Caspase Cascade Antibodies
Caspases play an essential role in apoptosis.
Caspase family proteases
All caspases (Cysteine Aspartate-specific Proteases) are synthesized in inactive forms, referred to as pro-caspases, that require dimerization/oligomerization and subsequent cleavage for activation.
Caspases are a family of proteases that play a central role in a host of processes, including cell death and inflammation. During apoptosis, a series of caspases (caspase-2, -8, -9 (Figure 1), -10 (Figure 2), -11, and -12) activate another group of downstream effector caspases (caspase-3, -6 (Figure 3), and -7) that cleave other proteins to complete the apoptotic program. Caspases-1 (Figure 4), -4, -5, -11, and -12 are involved in inflammation. Caspase-14 is not involved in apoptosis or inflammation but plays an important role in skin cell development.
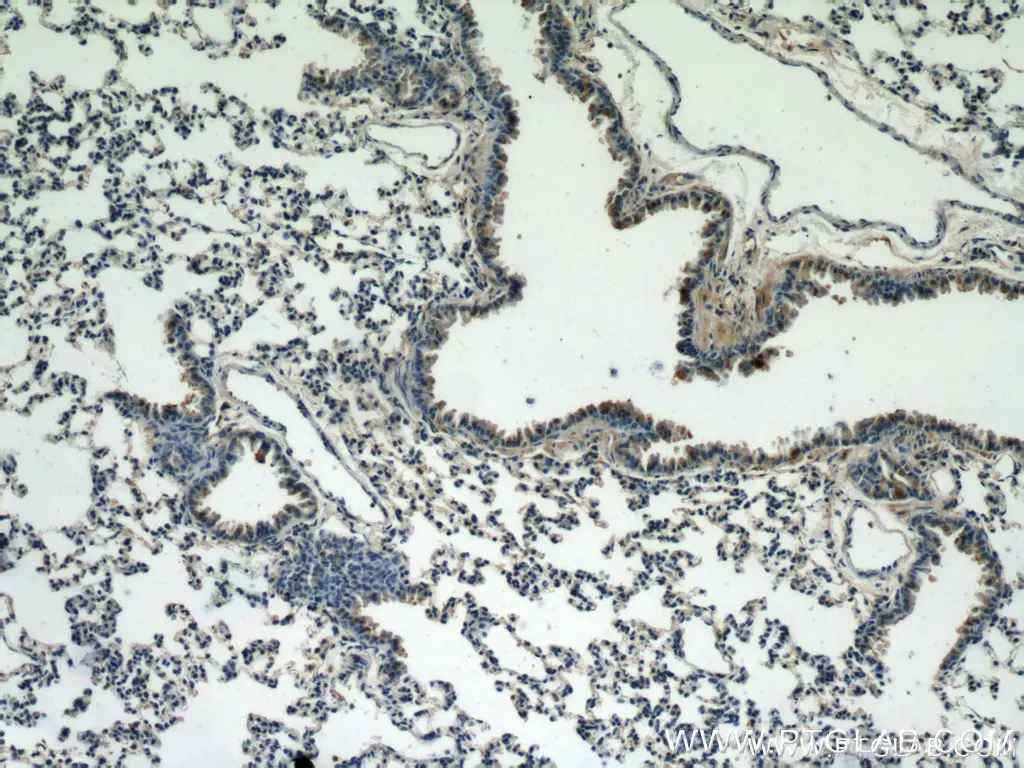
Figure 1. Immunohistochemistry of paraffin-embedded mouse lung tissue slide using 10380-1-AP (Caspase 9/p35/p10 antibody) at dilution of 1:25 (under 10x lens).
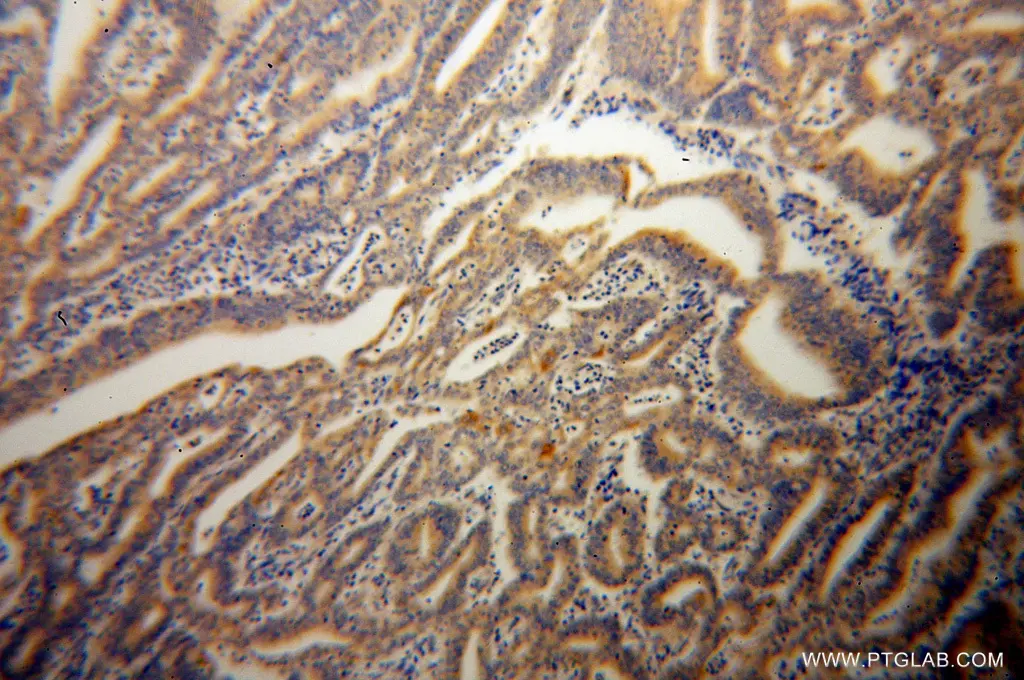
Figure 2. Immunohistochemistry of paraffin-embedded human endometrial cancer using 14311-1-AP (CASP10 antibody) at dilution of 1:100 (under 10x lens).
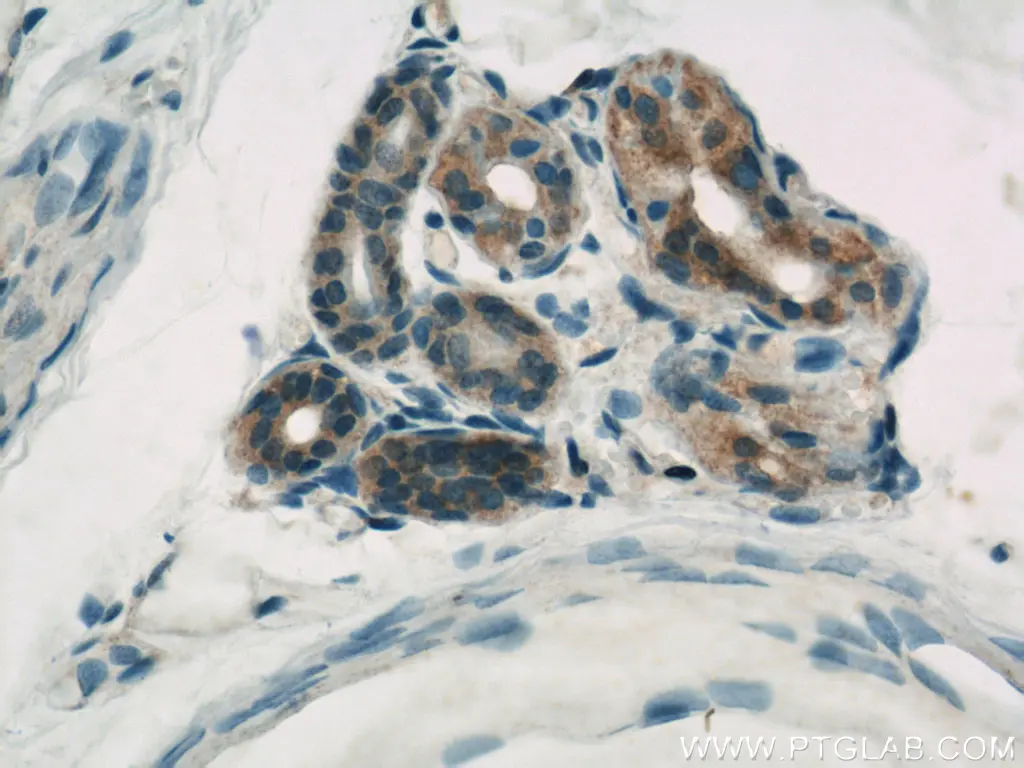
Figure 3. Immunohistochemistry of paraffin-embedded human skin using 10198-1-AP (CASP6 antibody) at dilution of 1:500 (under 40x lens).
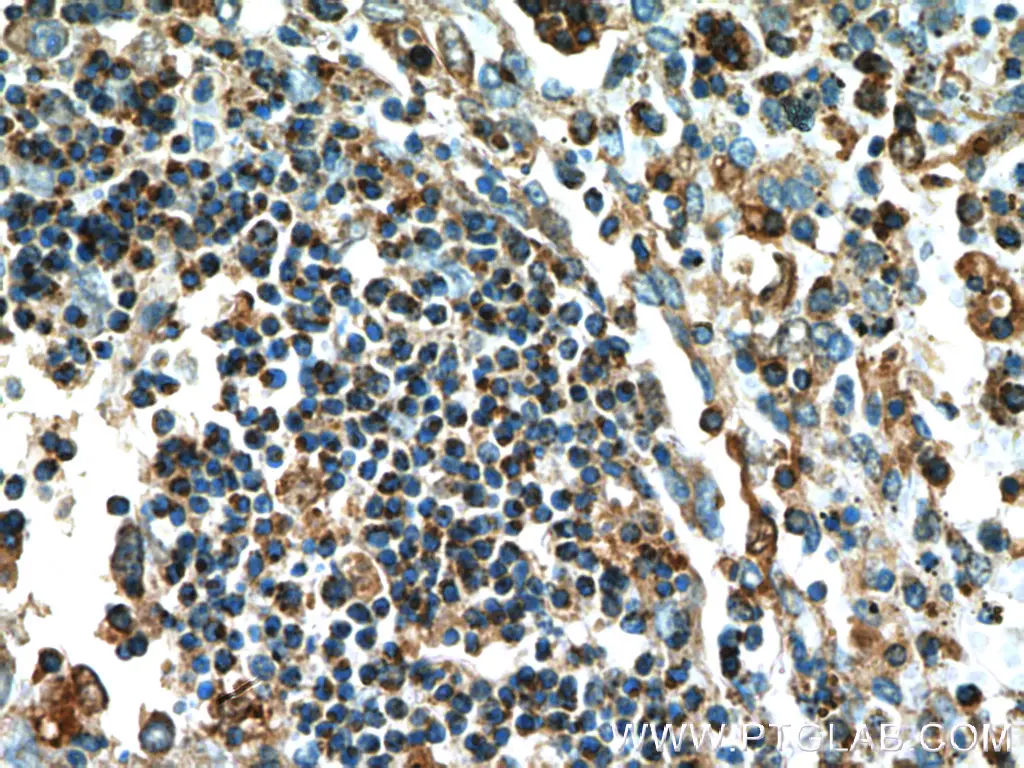
Figure 4. Immunohistochemistry of paraffin-embedded human spleen tissue slide using 22915-1-AP (Caspase 1 Antibody) at dilution of 1:200 (under 10x lens).
Distinct caspases pathways
Pro- and anti-apoptotic proteins are probably the first caspase targets following an apoptotic stimulus. Extrinsic or intrinsic death signals mediate caspase activation and apoptosis via two distinct pathways. The extrinsic pathway is triggered by ligand binding to cell surface death receptors, and the intrinsic pathway is activated by factors such as DNA damage, growth factor withdrawal, or loss of extracellular matrix contact and is mediated through the mitochondria.
Caspase-3 and caspase-8 in apoptosis
Caspases -3 and -8 are key regulators of the apoptotic response. Caspase-8 (Figure 5) is crucial for triggering apoptosis; an active caspase-8 cleaves caspase-3, which leads to cell death induction. Caspase-3 (Figure 6) has been identified as one of the key mediators of apoptosis. It cleaves Bcl-2 and Bcl-XL, which destroys the anti-apoptotic function of these proteins and releases C-terminal fragments that are pro-apoptotic. An acquired defect in apoptosis activation often leads to uncontrolled cell growth, oncogenesis, and cancer. Ligand-bound tumor necrosis factor (TNF) receptors initiate apoptosis by recruiting FADD and other death domain adaptor proteins that then recruit and activate caspases.
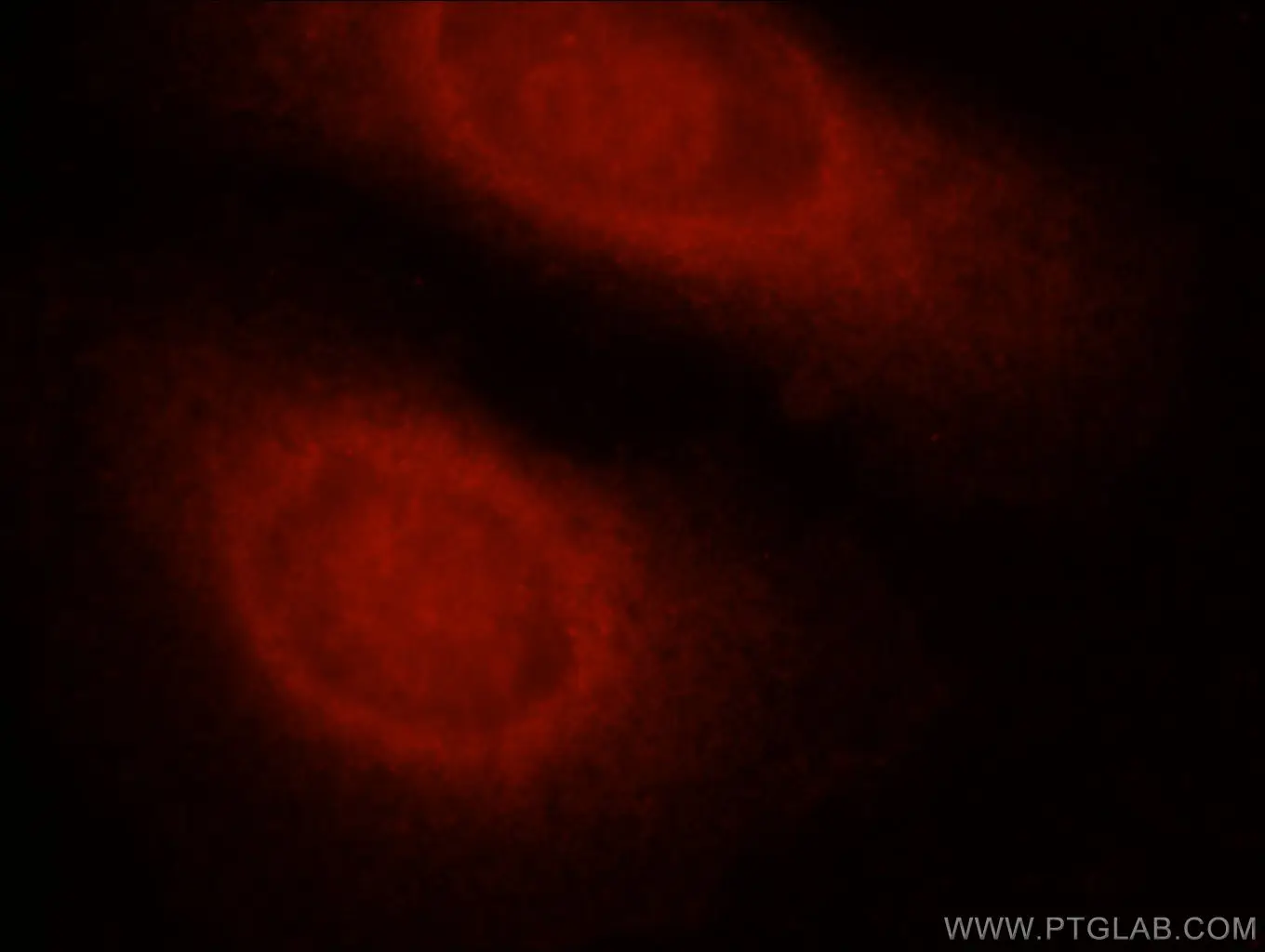
Figure 5. Immunofluorescent analysis of HepG2 cells using 13423-1-AP (Caspase 8 antibody) at dilution of 1:25 and Rhodamine-Goat anti-Rabbit IgG.
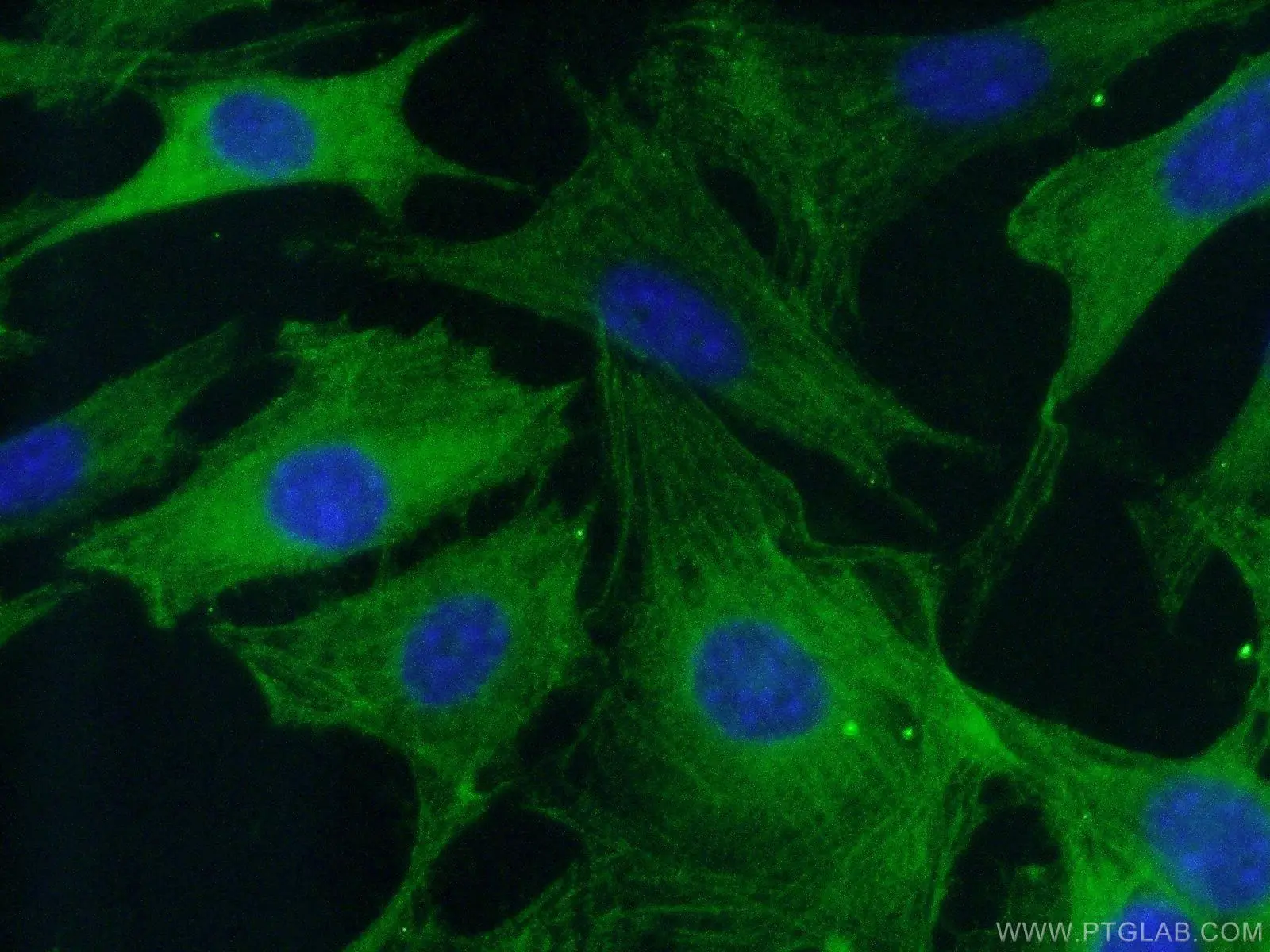
Figure 6. Immunofluorescent analysis of (-20℃ Ethanol) fixed NIH/3T3 cells using 19677-1-AP (Caspase 3 antibody) at dilution of 1:50 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG(H+L).
Related Content
What is the difference between necrosis and apoptosis?
Metabolic regulation of cell death
Cancer stem cells as a key to cure cancer
Molecular markers for liver cancer

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
