Introduction to cerebral organoids in research
Understand how brain organoids are made and why they are tipped to revolutionize life science research models.
Written by Ryszard Wimmer, PhD student at the Sorbonne Université in Paris
Organoids are taking the research world by storm due to their ability to model human organ development without the need for animal models. Starting from induced pluripotent stem cells, researchers have already been able to replicate complex physiological functions using organoids. Their future is bright, with huge potential to eventually create more complex and fully developed models. Proteintech has written this blog in collaboration with Ryzard Wimmer, a PhD student at Sorbonne Université in Paris, who works on cerebral organoid models. Below, we shed some light on the history and use of organoids in research and provide some tips and tricks for organoid culture and imaging.
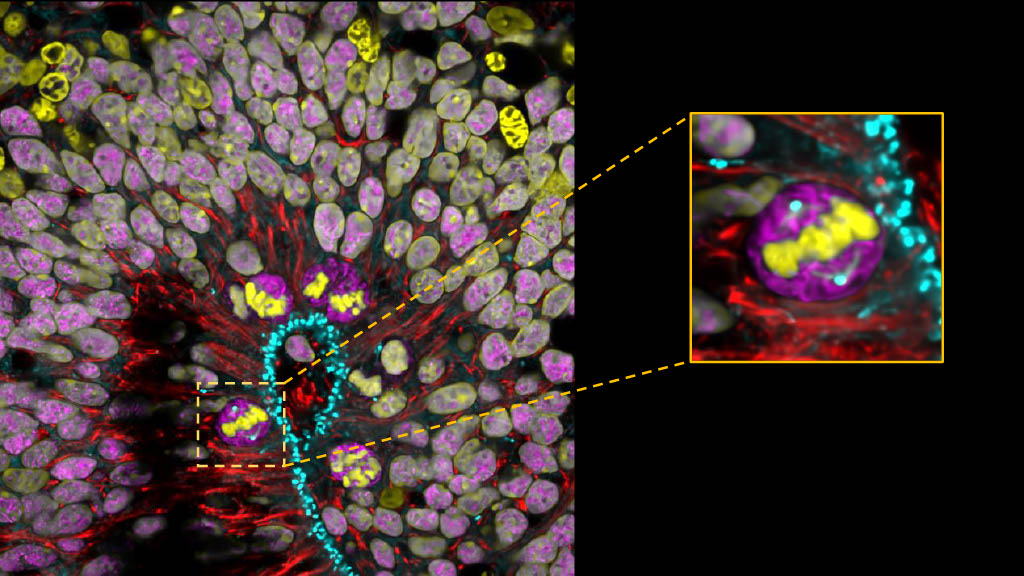
Human dorsal cerebral organoid at 7 weeks. 4% PFA fixed. Antibody dilution was 1:500 for Syne-2 (25265-1-AP) in Red. Apical Radial Glia cells perform Interkinetic Nuclear Migration and divide at the ventricular-like zone of the cerebral organoid. Zoomed square: aRG cell in metaphase with an established mitotic spindle. Image by Wimmer Ryszard, from the Institut Curie in Paris, France. .
What are organoids used for?
Historically, animal models such as mouse models have been challenging to tackle certain questions when it comes to human development and disease. Complications in using animal are well known, including the fact that they do not best represent human tissue or disease. To tackle these issues, scientists have developed ways of generating 3D model systems derived from human stem cells. As defined by the Harvard Stem Cell Institute, “Organoids are tiny self-organized three-dimensional tissue cultures that are derived from stem cells. Such cultures can be crafted to replicate much of the complexity of an organ, or to express selected aspects of it like producing certain types of cell types”1. Thus, organoids recapitulate the cellular architecture of an in-vivo tissue and some of its functions. Currently, organoids are not representative of the true functioning organ due to their lack of physiological inputs such as blood vessels, hormones and interaction with other organs. The use of organoids enables basic research into the development of human organs from embryonic to post-natal stages in response to different treatments or physiological inputs. Organoids are an excellent model to help understand basic cell biology at a level more complex than that of primary cell lines, providing greater insight to the representation of an in-vivo tissue. In the future, researchers are hopeful that organoids could be used to treat certain tissue injuries or cancer.
What are organoids made of?
In the mid 2000s, Shinya Yamanka and his team made the landmark discovery of how to create induced pluripotent stem cells (iPS cells). They found that skin cells could be reversed to an embryonic stem cell like state by expressing certain genes that are normally silenced in somatic cells like fibroblasts2. Organoids are made using these iPSCs, which are differentiated into cells from the three primary germ layers: endoderm, mesoderm, and ectoderm. iPSCs can be cultured as single cells or colonies by embedding them in different matrices with cocktails of differentiation factors, and thus can transform into the three embryonic layers. Currently, the best characterized and most used organoids are intestinal, heart, muscle, and cerebral (brain) organoids.
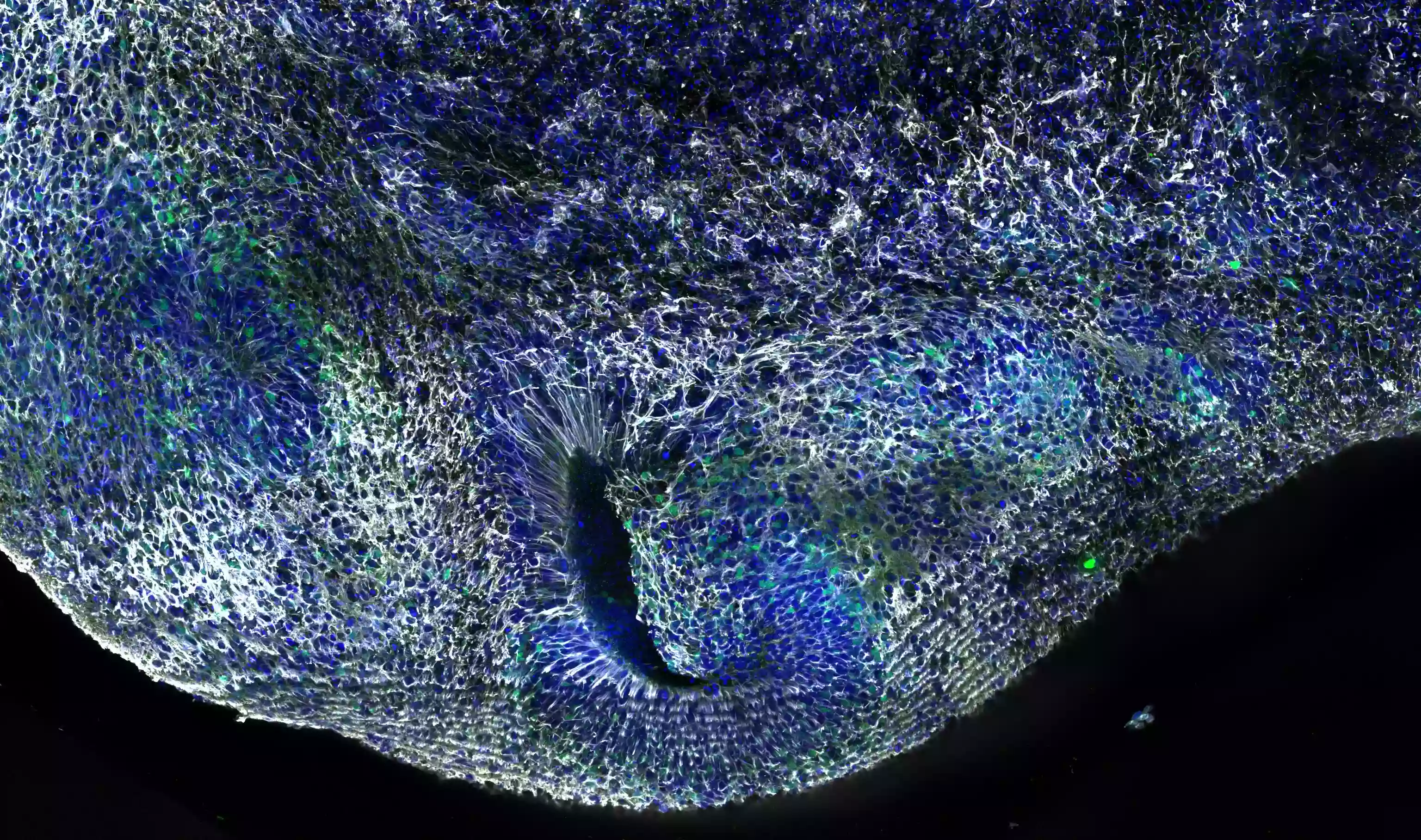
Human dorsal cerebral organoid at 12 weeks. 4% PFA fixed. Antibody dilution was 1:500 for Syne-2 (25265-1-AP; white), DAPI 1:1000. Image by Wimmer Ryszard from the Institut Curie in Paris, France.
Different types of brain organoids
The emergence of sequencing techniques applied to the field of cortical development has enabled the discovery of many factors that can determine the specific fate of organoids. Thus, using a cocktail of transcription factors we can obtain different organoids that closely resemble the architecture of a desired brain region. This is demonstrated well by Dr. Sergiu Pasca’s group at the University of Stanford, who generated models of different organoids such as the dorsal cerebral organoids, spinal cord, and muscle organoids. Amazingly, they were able to assemble these 3 model systems into an “assembloid” which can simulate muscle contraction from the cerebral cortex3. Another exciting example comes from the lab of Madeline Lancaster at Cambridge University, who created choroid plexus organoids capable of secreting cerebral spinal fluid (CSF)4.. These functional models highlight the vast potential of what brain organoids can model and achieve by theoretically applying the correct cocktail of factors. The ability to accurately model human neural systems, neurodevelopment conditions and diseases using brain organoids will be of huge relevance to the neuroscience field.
Cerebral organoids protocols and maintenance
Like many protocols from western blotting to patch clamp recordings, cerebral organoid protocols are varied and often specific to individual labs. As a starting point, the Lancaster Protocol4 or the protocol from Dr. Sergiu Pasca are popular and well used by many labs5. The most important point for organoid protocols is to start with a healthy population iPS cells. These cells are very delicate and must be maintained in specific conditions. For example, even slight changes in carbon dioxide levels, humidity, temperature, or culture media may cause variability in your organoid culture.
The size of your organoid cultures is also important for long-term viability. Overtime, as the organoid increases in volume, a necrotic centre starts to form due to oxygenation issues6. Thus, slicing every couple of weeks is recommended to help prevent this and maintain healthy cultures.
For a more information on cerebral organoid culture and tips, watch our webinar by Michela Barabato, a Senior Research Technician at the University of Edinburgh. Watch here!
Tips for imaging organoids
Preparation of organoids for imaging depends on the type of imaging required for your specific experiment. For example, thick slices are required for live imaging, whereas for immunofluorescence, it is better to have thinner, fixed slices enabling better antibody penetration. With live tissue imaging, it helps to have microscope objectives with a large working distance. Using a wide spinning disc microscope over a confocal can help in this regard and can also save time. To help see details from each cell type, using 40x and 60x objectives are recommended. There are many well documented tips for improving immunofluorescence images, but one that deserves to be repeated is to increase the number and length of your wash steps. The more you wash your samples, the better your images will be!
HumanKine® growth factors for organoid culture
For healthy maintenance of human iPSC culture, using high quality and trusted reagents is key. HumanKine® recombinant cytokines and growth factors are authentic human proteins that are HEK293 cell expressed and demonstrate greater activity than E. coli-expressed proteins. They are also tag and endotoxin-free.
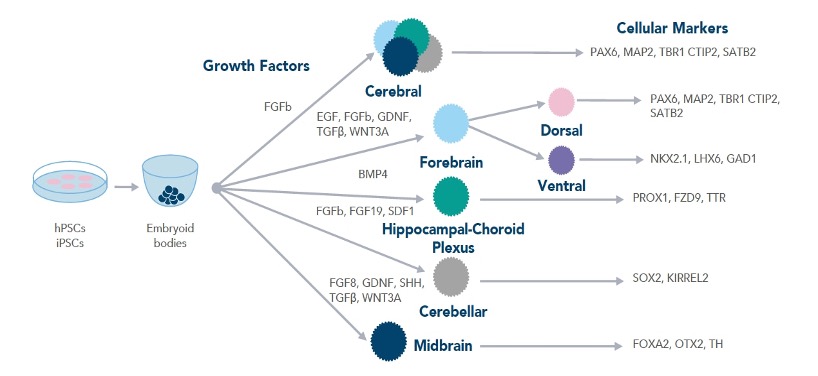
Workflow demonstrating the growth factors required for different types of brain organoids
HumanKine® Growth Factors for organoid generation
Target |
Purity |
Activity (EC50) |
Source |
Model Organoid |
GMP-grade? |
| BMP4 | >95% |
1.5-9 ng/mL |
HEK293 |
Hippocampal-Choroid Plexus |
Yes |
| EGF | >95% |
Active |
HEK293 |
Cortical |
|
| FGFb | >95% |
0.05-0.4 ng/mL |
HEK293 |
Cortical, Cerebellar |
Yes |
| FGF8 | >95% |
≤ 10 ng/mL |
HEK293 |
Midbrain |
|
| GDNF | >95% |
≤ 10 ng/mL |
HEK293 |
Midbrain, Hypothalamus, Forebrain |
|
| SHH | >90% |
≤ 350 ng/mL |
HEK293 |
Midbrain |
|
| TGF | >95% |
≤ 0.5 ng/mL |
HEK293 |
Midbrain, Hypothalamus, Forebrain |
Yes |
| WNT3A | >90% |
2-17 ng/mL |
HEK293 |
Midbrain, Hypothalamus, Forebrain |
Yes |
Antibodies for brain organoid characterization
Target |
Cell/Tissue Type |
Target |
Cell/Tissue Type |
|
Deep-layer cortical neurons |
ventral forebrain |
||
|
Developing neurons |
Midbrain cells |
||
|
NSCs, forebrain |
Radial glia/NSCs |
||
|
Forebrain NSCs |
Hippocampal neurons |
||
|
Intermediate progenitors |
Pre-plate/Cajal-retzius cells |
||
|
Midbrain dopaminergic neurons |
Cortical neurons across corpus callosum |
||
|
Forebrain cells |
Radial glia/NSCs |
||
|
Cortical precursors, hippocampal neurons |
Pre-plate/Cajal-retzius cells |
||
|
Ventral forebrain |
Midbrain NSCs |
||
|
Cerebellar progenitors |
Choroid plexus |
||
|
Ventral forebrain |
Neurons |
||
|
NSCs, migrating neurons, neural plate |
GABAergic and glycinergic neurons |
||
|
NSCs, neurons |
Radial glia/NSCs |
||
|
NSCs, progenitors GABAergic interneurons, oligodendrocytes |
References
- https://hsci.harvard.edu/organoids
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861-72. doi: 10.1016/j.cell.2007.11.019. PMID: 18035408.
- Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, Vogel H, Fan HC, Paşca SP. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 2020 Dec 23;183(7):1913-1929.e26. doi: 10.1016/j.cell.2020.11.017. Epub 2020 Dec 16. PMID: 33333020; PMCID: PMC8711252.
- Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 2020 Jul 10;369(6500):eaaz5626. doi: 10.1126/science.aaz5626. Epub 2020 Jun 11. PMID: 32527923; PMCID: PMC7116154.
- Sloan SA, Andersen J, Pașca AM, Birey F, Pașca SP. Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 2018 Sep;13(9):2062-2085. doi: 10.1038/s41596-018-0032-7. PMID: 30202107; PMCID: PMC6597009.
- Sloan SA, Andersen J, Pașca AM, Birey F, Pașca SP. Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 2018 Sep;13(9):2062-2085. doi: 10.1038/s41596-018-0032-7. PMID: 30202107; PMCID: PMC6597009.
Related Content
Tips and techniques for culturing brain organoids
7 tips to successfully culture primary rodent neurons
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.