Understanding FGF Signaling: A Key Pathway in Development and Disease
Written by Jaime Fernández Sobaberas, 3rd year PhD student in Biochemistry at Heidelberg University, Germany.
Introduction to FGF Signaling
The fibroblast growth factor (FGF) signaling pathway plays a pivotal role in regulating various cellular processes such as cell proliferation, differentiation, migration, and survival1. It is a highly conserved pathway present across diverse species, from invertebrates to vertebrates2. The FGF signaling pathway consists of a family of growth factors, their receptors, and downstream intracellular signaling molecules.
Components of FGF Signaling
- FGF Ligands: The mammalian FGF family comprises 22 ligands designated FGF-1 through FGF-23 (with the exception of FGF-15). They exert their biological functions by binding to specific FGF receptors.
- FGF Receptors (FGFRs): There are four known FGFRs (FGFR1-4) in humans, which are transmembrane receptor tyrosine kinases. Upon ligand binding, FGFRs undergo dimerization and autophosphorylation, leading to the activation of downstream signaling cascades.
- Downstream Signaling Pathways: Activation of FGFRs initiates several downstream signaling pathways, including the Ras-MAPK pathway, PI3K-Akt pathway, and PLCγ pathway. These pathways regulate gene expression, cytoskeletal dynamics, and other cellular functions.
FGFs induce dimerization, kinase activation, and transphosphorylation of tyrosine residues of FGFRs, leading to activation of downstream signaling pathways. Signal transduction largely follows one of three pathways. The RAS/MAP kinase pathway, initiated upon the formation of an FRS2 complex, controls cell proliferation and differentiation. The PI3/AKT pathway is also initiated by the formation of an FRS2 complex and regulates cell survival and fate determination. Finally, upon binding of PLC to the activated FGFR, DAG and IP3 are formed, activating PKC, which influences cell morphology, migration, and adhesion.
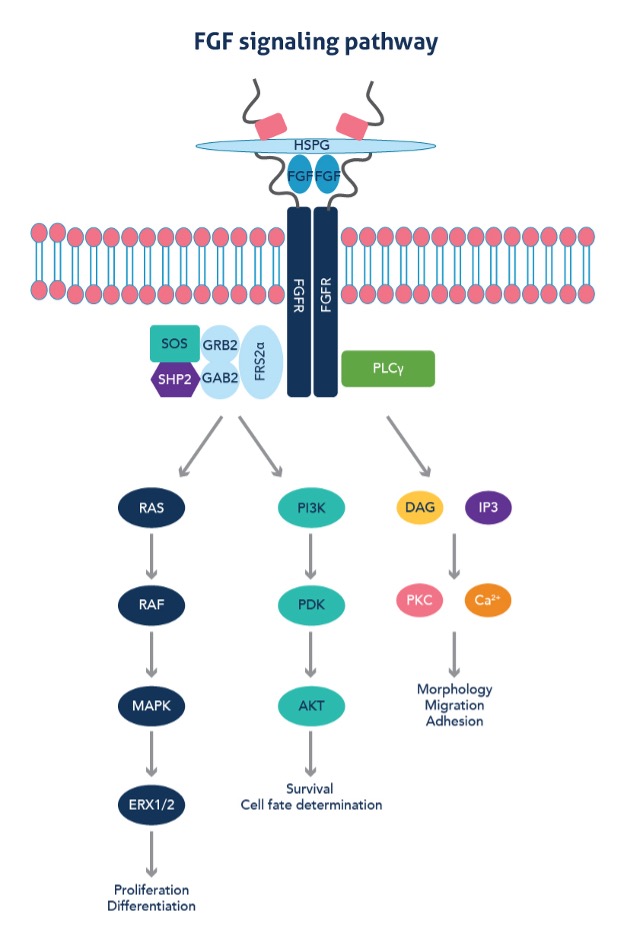
Figure 1: FGF signaling pathways play crucial roles in the regulation of proliferation, differentiation, and apoptosis via downstream effectors. Adapted from Teven et al, 20143.
Functions of FGF Signaling
- Embryonic Development: FGF signaling is crucial for embryonic development, including gastrulation, organogenesis, and limb development. It regulates cell fate determination, morphogenesis, and tissue patterning during embryogenesis.
- Tissue Repair and Regeneration: FGF signaling plays a critical role in tissue repair and regeneration processes such as wound healing and bone regeneration. It promotes cell proliferation and migration at the site of injury, facilitating tissue remodeling.
- Angiogenesis: FGF signaling is involved in angiogenesis, the process of new blood vessel formation. FGFs stimulate endothelial cell proliferation and migration, promoting the formation of new blood vessels in physiological and pathological conditions.
Role of FGF Signaling in Cancer
FGF signaling plays a significant role in the development and progression of cancer. Dysregulation of the FGF signaling pathway may upregulate downstream cell signaling and drive tumorigenesis. Activating mutations lead to ligand-independent receptor dimerization and activation or constitutive activation of the kinase domains. The amplification of FGF/FGFR genes results in protein overexpression, which may contribute to enhanced FGFR-mediated cell signaling. Chromosomal translocations lead to the formation of fusion proteins that may cause receptor activation independent of FGF signaling. These mechanisms lead to the hyperactivation of downstream signaling such as the Ras-MAPK and PI3K-Akt pathways. Additionally, FGF signaling can induce epithelial-mesenchymal transition (EMT), a process by which cancer cells acquire invasive and metastatic properties, contributing to tumor spread and treatment resistance. Therefore, dysregulation of the FGF signaling pathway drives cancer progression through uncontrolled cell division, apoptosis avoidance, new blood vessel formation, and epithelial-mesenchymal transition.
Hence, targeting FGF signaling has emerged as a promising therapeutic strategy for cancer treatment. These targeted therapies hold potential for improving patient outcomes by specifically blocking the oncogenic effects of dysregulated FGF signaling while sparing normal cells.
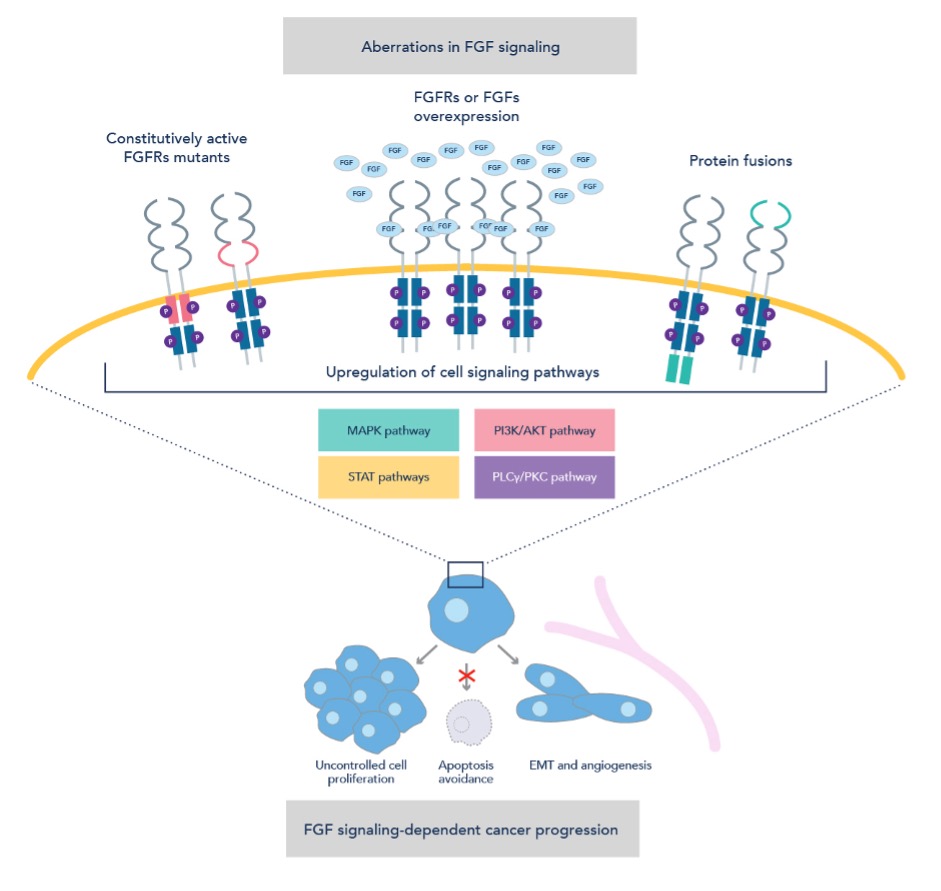
Figure 2: FGF signaling pathway mechanisms in cancer progression. Adapted from Szymczyk et al, 20214.
Therapeutic Targeting of FGF Signaling in Cancer
Given the central role of FGF signaling in cancer progression, targeting this pathway has become a focus for developing novel cancer therapies. Various therapeutic strategies have been employed, including FGFR inhibitors, monoclonal antibodies, and ligand traps.
- FGFR Inhibitors: FGFR inhibitors are small molecules designed to block the kinase activity of FGFRs, preventing the activation of downstream signaling pathways. Several FGFR inhibitors have been developed and are undergoing clinical trials for the treatment of various cancers, including lung cancer, breast cancer, and bladder cancer. These inhibitors can be classified into two main categories: selective and non-selective FGFR inhibitors.
- Monoclonal Antibodies: Monoclonal antibodies targeting FGFRs are being developed to block ligand-receptor interactions, thereby inhibiting receptor dimerization and activation.
- FGF Ligand Traps: Ligand traps are another approach that aims to sequester FGF ligands, preventing them from binding to FGFRs. These agents neutralize circulating FGFs, disrupting autocrine and paracrine loops that fuel cancer growth.
Other Therapeutic Implications of FGF Signaling
- Regenerative Medicine: Manipulating FGF signaling holds potential for regenerative medicine applications. FGFs have been investigated for their ability to promote tissue repair and regeneration in various contexts, including wound healing, myocardial infarction, and neurodegenerative diseases.
- Metabolic Disorders: Modulating FGF signaling pathways offers therapeutic avenues for treating metabolic disorders such as obesity and type 2 diabetes. FGF19 and FGF21 are involved in regulating bile acid metabolism, glucose homeostasis, and lipid metabolism. Thus, FGF21 analogs and agonists are being explored as potential treatments to improve insulin sensitivity and lipid metabolism.
Conclusion
In summary, FGF signaling is a highly conserved pathway that regulates diverse cellular processes essential for development, tissue homeostasis, and disease. Understanding the molecular mechanisms underlying FGF signaling provides insights into its roles in physiology and pathology, offering potential therapeutic opportunities for a range of diseases. Targeting FGF signaling has opened up new therapeutic avenues, with FGFR inhibitors, monoclonal antibodies, and ligand traps offering promising strategies for treating cancers with FGFR alterations. While significant progress has been made, ongoing research is needed to uncover new therapeutic targets and strategies for addressing unmet medical needs in various fields, from cancer therapy to regenerative medicine and metabolic disorders.
Native FGF Proteins and primary antibodies for FGF proteins
|
Target |
||
|
FGF1 |
||
|
FGF2 |
||
|
FGF3 |
|
|
|
FGF4 |
|
|
|
FGF5 |
|
|
|
FGF7 |
||
|
FGF8 |
||
|
FGF9 |
||
|
FGF10 |
||
|
FGF12 |
|
|
|
FGF13 |
|
|
|
FGF16 |
|
|
|
FGF17 |
|
|
|
FGF18 |
|
|
|
FGF21 |
|
References
- Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, Ni Z, Zhang B, Zhang D, Luo F, Chen H, Sun X, Feng JQ, Qi H, Chen L. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020 Sep 2;5(1):181. doi: 10.1038/s41392-020-00222-7. PMID: 32879300; PMCID: PMC7468161.
- Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):215-66. doi: 10.1002/wdev.176. Epub 2015 Mar 13. PMID: 25772309; PMCID: PMC4393358.
- Teven CM, Farina EM, Rivas J, Reid RR. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014 Dec 1;1(2):199-213. doi: 10.1016/j.gendis.2014.09.005. PMID: 25679016; PMCID: PMC4323088.
- Szymczyk J, Sluzalska KD, Materla I, Opalinski L, Otlewski J, Zakrzewska M. FGF/FGFR-Dependent Molecular Mechanisms Underlying Anti-Cancer Drug Resistance. Cancers (Basel). 2021 Nov 18;13(22):5796. doi: 10.3390/cancers13225796. PMID: 34830951; PMCID: PMC8616288.
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. Epub 2001 Mar 9. PMID: 11276432; PMCID: PMC138918.
- Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3441-5. doi: 10.1073/pnas.88.8.3441. PMID: 1707542; PMCID: PMC51463.
Related Content
Cancer and metastasis product focus
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

