What is Macrophage Polarization?
Written by Consuelo Coser, PhD Student at University of Nottingham
Macrophage polarization is a process of the immune response whereby specific environmental cues induce monocyte differentiation into distinctly functional macrophage phenotypes: M1 and M2. These polarized cells can combat different threats to the host.
THE IMMUNE RESPONSE
The purpose of the immune system is to protect the body from antigens, which can endanger the host’s health. It involves both innate and adaptive immune responses, and their co-operation aims to eliminate pathogenic organisms, neutralize toxins or allergens, and counter anything potentially harmful.
The innate immune system
The innate immune system is the body’s first line of defense against pathogens. It includes physical and physiological barriers such as the skin, temperature, bioactive molecules, and membrane receptors/cytoplasmic proteins that target specific molecular patterns on the surface of pathogens. The innate immune system activates promptly when encountering antigens and is characterized by non-specific mechanisms of defense and the absence of immunologic memory.
The adaptive immune system
Unlike the innate system, the adaptive immune response is characterized by a time lag from the pathogen invasion, as it involves the following steps: recognition of specific antigens, the distinction between self and non-self antigens, and the development of an antigen-specific response. Additionally, the adaptive immune system shows immunologic memory, enabling a faster and more effective response upon subsequent exposures to the same antigen1; 2.
THE ROLE OF MACROPHAGES
Macrophages are effector cells of the innate immune system, and they play a major role in the immune response. They are scavenger cells responsible for removing debris, dead cells, and damaged tissues through phagocytosis and degradation. They phagocytose anything recognized as non-self, such as bacteria and implants; the latter must be designed to resist this action via size and material properties3. Macrophages can act as both pro- and anti-inflammatory responders due to their ability to polarize into distinct phenotypes. They can secrete both pro- and anti-inflammatory cytokines, and antimicrobial mediators such as reactive oxygen species (ROS), nitric oxide (NO), and myeloperoxidase4.
How are macrophages polarized?
Macrophages are derived from circulating monocytes that differentiate upon migration into the tissue where they are needed. Depending on the environment and the stimuli they are subjected to, monocytes differentiate into the following routes that lead to different macrophage polarization statuses known as M0, M1, and M2. Among the various cytokines and growth factors that affect macrophage phenotype, macrophage colony-stimulating factor (M-CSF) plays a pivotal role as it regulates the proliferation, differentiation, and functional activation of monocytes.
The M0 phenotype refers to naïve macrophages, namely macrophages that have not been exposed to either pro- or anti-inflammatory stimuli.
Classically activated macrophages, commonly known as M1 phenotype, are induced by lipopolysaccharides (LPS), interferon γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, IL-12, IL-18, TNF-α, and TNF-β. They are pro-inflammatory cells that produce and release pro-inflammatory cytokines, reactive nitrogen and oxygen intermediates 5, enzymes, and acids 6 such as lysozyme and neutral proteinases.
The alternative activation route leads to the M2 phenotype. M2 macrophages release anti-inflammatory factors such as IL-10, matrix metalloproteinases, and growth factors including vascular endothelial growth factor (VEGF) 7, TGF-β1, and PDGF 8. Additionally, M2 macrophages are characterized by a higher phagocytic activity. Cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13, as well as glucocorticoids, stimulate the M2 phenotype.
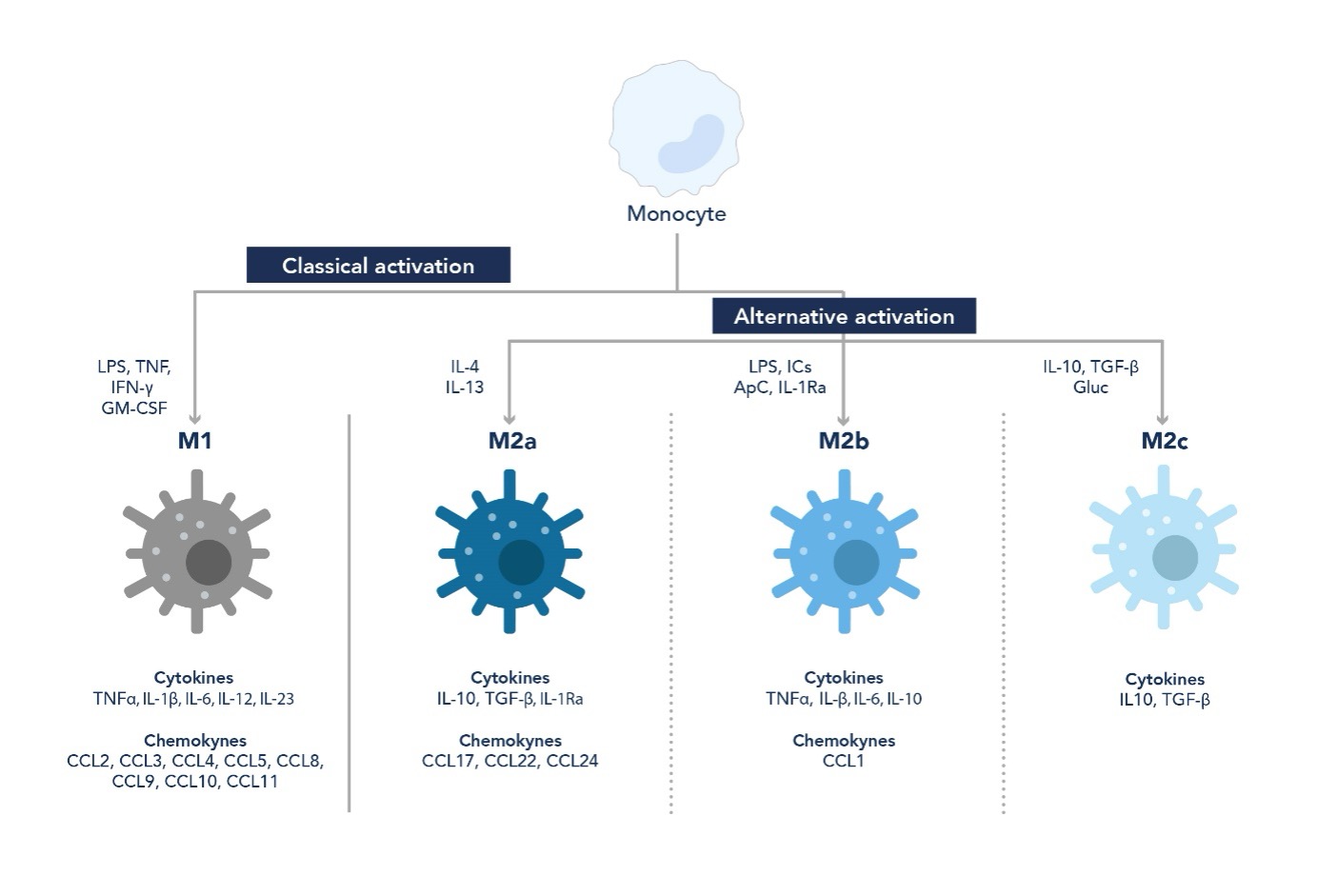
Figure 1. Differentiation pathways from monocytes to macrophages. Depending on the stimuli they are subjected to, macrophages can show either pro-inflammatory (M1) or anti-inflammatory (M2) behavior.
Despite being divided into these two main classes, macrophages can show different sub-phenotypes depending on their surrounding microenvironment. Macrophages are highly plastic and dynamic cells, and their phenotype can change over time in response to various stimuli 9.
MACROPHAGE RESEARCH: THE EXPERIMENTAL WORKFLOW
Research on macrophages is critical due to their role in the immune response and diseases such as the foreign body reaction. The following sections explore the workflow of monocyte isolation and differentiation, as well as macrophage characterization.
Monocyte isolation
Monocytes can be isolated from whole blood samples through positive or negative selection. Positive selection involves targeting a specific surface antigen to select the desired cell type. For example, CD14 is a surface antigen expressed on monocytes, therefore Human CD14 Magnetic Beads can be used to isolate these cells 10. On the other hand, negative selection involves removing unwanted cells until monocytes remain. This method requires the use of different Magnetic Cell Separation Kits.
Monocyte differentiation protocol into M0, M1, and M2 macrophages 10
Note: all steps must be carried out using aseptic techniques under sterile conditions. The number of cells and volume of media used in this protocol refers to monocyte differentiation performed in a 24-well plate.
Materials: RPMI-1640 medium supplemented with FBS (10% v/v), L-Glutamine (1% v/v), Penicillin-Streptomycin (1% v/v); M-CSF, GM-CSF, IFN-g, IL-4.
Protocol:
Day 1
- Resuspend monocytes (3.5 – 6x106) isolated from healthy blood donor in media (12 mL)
- Split the cell suspension into three falcon tubes (4 mL each), one for each differentiation pathway
- Add the following cytokines (Table 1) to each falcon tube, depending on the desired phenotype
Table 1. Cytokine Components. To differentiate monocytes into different macrophage phenotypes, complete RPMI-1640 medium is supplied with cytokines, according to the desired phenotype.
|
Phenotype |
Cytokine |
Final concentration (ng/mL) |
|
M0 |
10 |
|
|
M1 |
20 |
|
|
20 |
||
|
M2 |
50 |
|
|
20 |
Note: the volume of cytokine to add depends on the concentration of their stock solution. To calculate the volume needed use the formula: Ci x Vi = Cf x Vf
- Gently mix and seed into a 24-well plate (1 mL/well)
- Incubate (37°C, 5% CO2) until Day 3.
Day 3
- Change media (use same quantities of media and cytokines for Day 1)
- Incubate (37°C, 5% CO2) until Day 7.
Macrophage characterization
On Day 7, the differentiation process is complete. Macrophages can be fixed (PFA 4%, 15 minutes) and characterized using different validation techniques.
a. Morphology
As shown in Figure 2, macrophage morphology changes based on the phenotype. Naïve macrophages (M0) are characterized by a round shape, unlike M1 and M2 phenotypes. M1 shows an elongated morphology, whereas M2 phenotype tends to form round clusters.
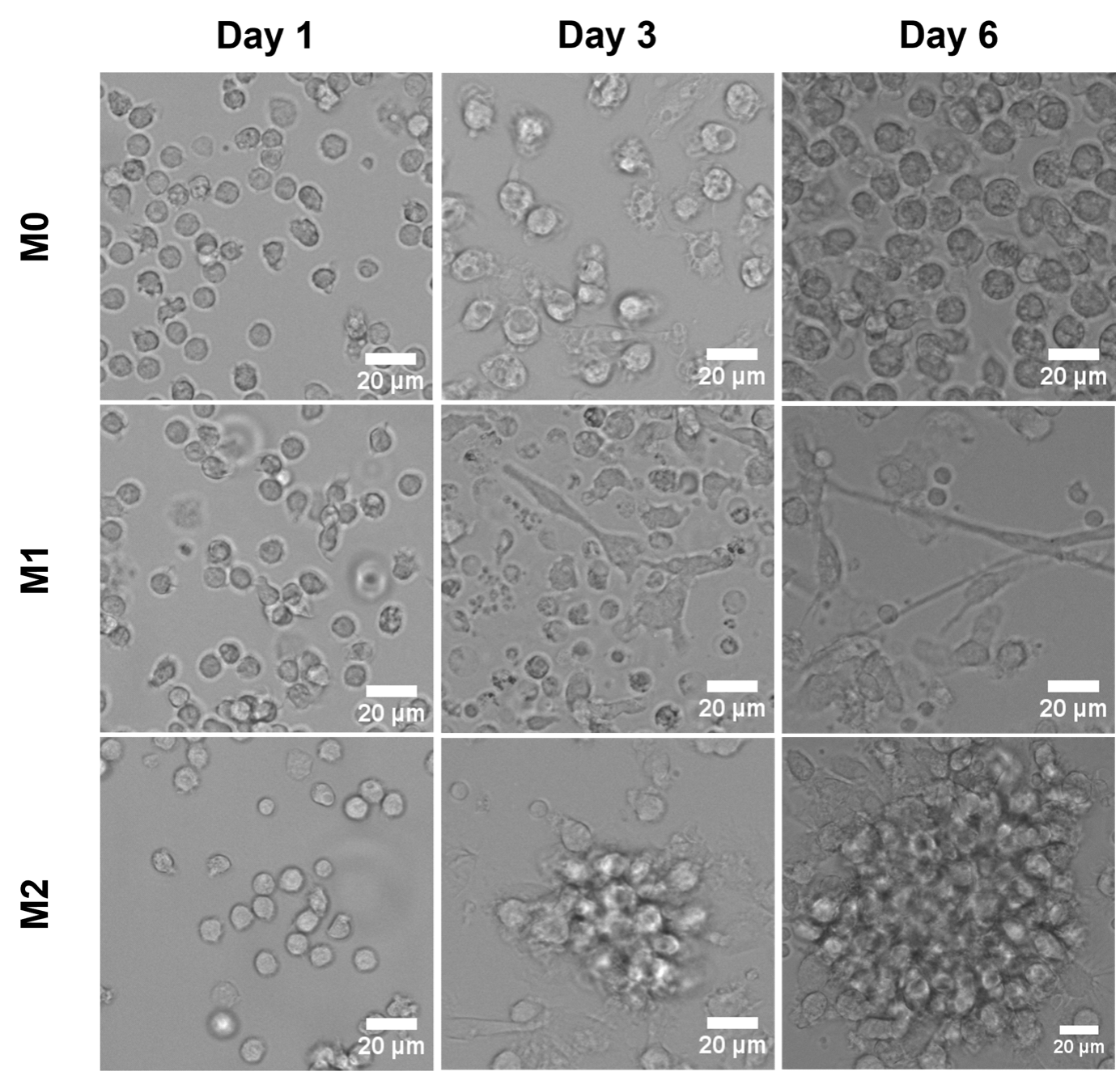
Figure 2. Images of monocytes (Day 1 and Day 3) and macrophages (Day 6) cultured on tissue culture plastic and differentiated into M0, M1, and M2. Scale bars: 20 µm.
b. Immunofluorescent staining
M1 and M2 differentially express calprotectin and mannose receptor (MR): calprotectin is an M1 marker, while MR is an M2 marker.
Anti-calprotectin and anti-MR antibodies with different fluorescent dyes can be used to characterize macrophage phenotypes through immunofluorescent imaging.
It is important to maintain a similar number of cells in the images undergoing fluorescent quantification.
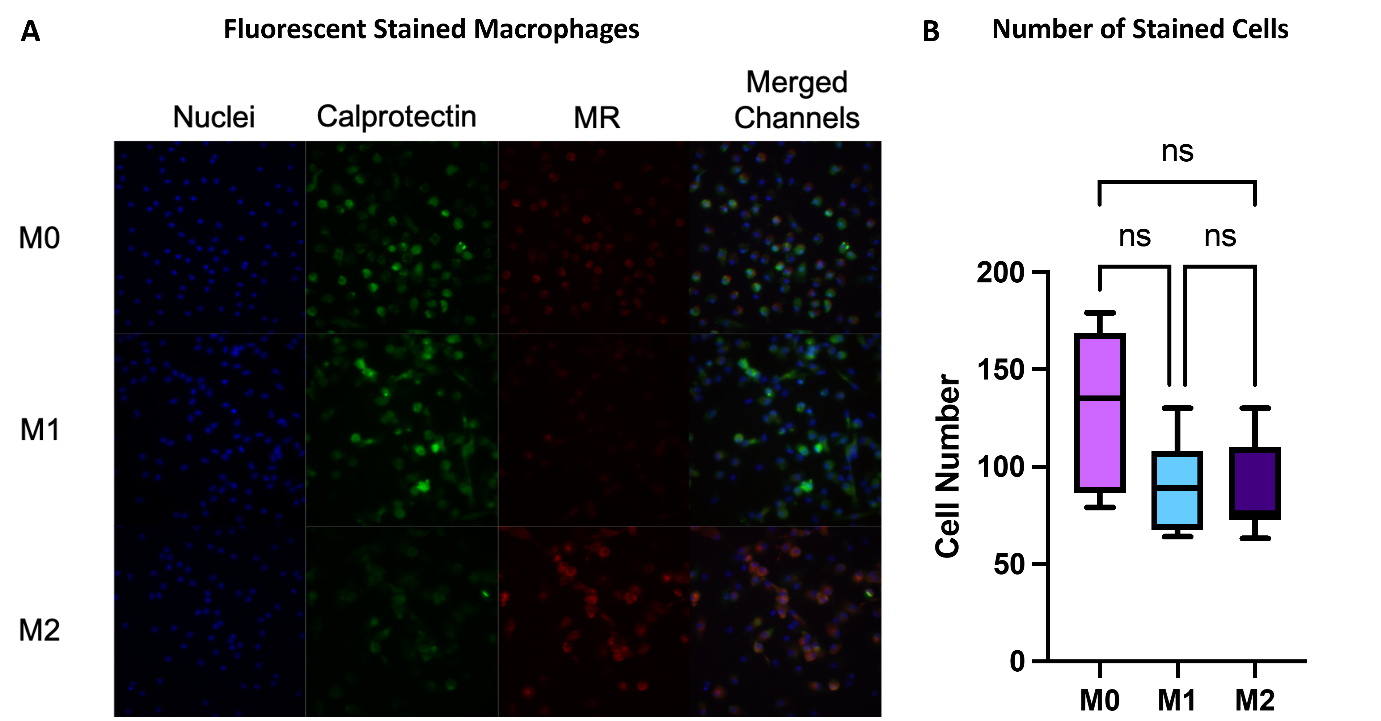
Figure 3. (A) Fluorescent images of cells stained for calprotectin (green), MR (red), and nuclei (DAPI) taken on blue (359 (540 nm) channels, and merged. (B) Number of cells present in each image. Data is presented as mean of triplicates ± s.d.
c. Quantification of pro- and anti-inflammatory cytokines
Media can be collected to quantify the cytokines secreted from the cells. Using different ELISA Kits, it is possible to determine the cytokine profile of macrophages, and therefore their phenotype. Figure 4 shows an example of cytokine quantification: the pro-inflammatory cytokines TNF-α and IL-6 were used as pro-inflammatory markers, whereas IL-10 and CCL18 were taken as anti-inflammatory markers, and their concentrations in the media were measured.
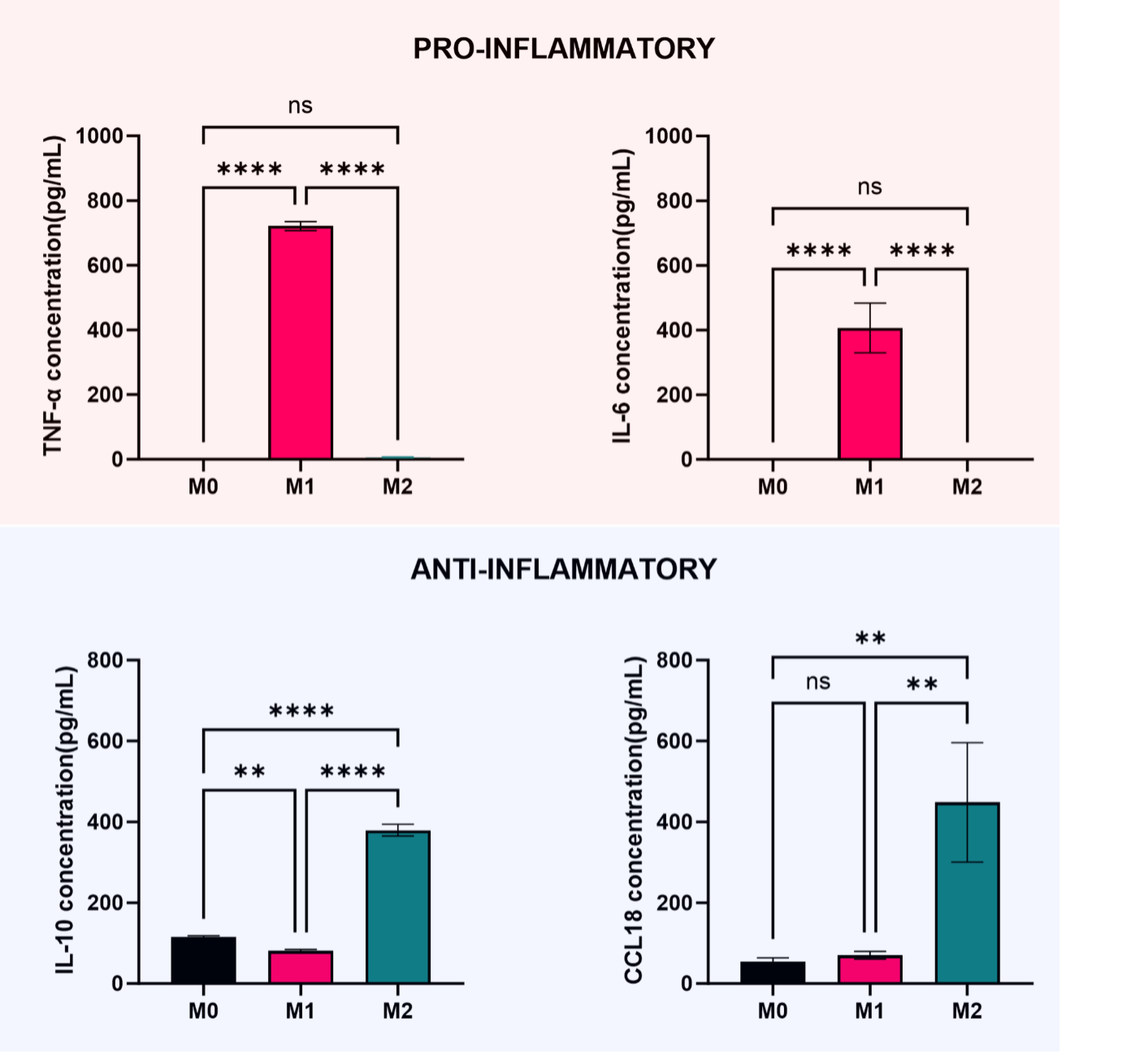
Figure 4. Quantification of cytokines TNFa, IL-6, IL-10, and CCL18 secreted in the culture media by macrophages. Data is presented as mean of triplicates ± s.d.
CONCLUSION
In conclusion, macrophages play a pivotal role in maintaining homeostasis by destroying infectious organisms, clearing cellular debris, and wound healing. However, they can also stimulate tumor growth and the foreign body reaction.
Therefore, understanding macrophage polarization and its underlying mechanism is crucial for developing strategies to support the positive actions of these immune cells while limiting their negative aspects.
Related products:
|
Target |
||||
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
|
|
|
|
✔️ |
✔️ |
✔️ |
✔️ |
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
|
|
|
|
✔️ |
|
|
|
|
|
✔️ |
|
|
|
Author Biography:
|
I graduated from the University of Milan (Italy) in 2020 with a Master in Pharmaceutical Chemistry and Technology (110/100 cum Laude). In my final year, I carried out a research project in medicinal chemistry, that consisted in the design and synthesis of covalent GAPDH inhibitors as potential antimalarial drugs. Before starting PhD, I qualified as a pharmacist in Italy, and I worked as a Patent Attorney Trainee in the pharmaceutical field. I am currently in my second year of PhD at the University of Nottingham as part of the EPSRC-SFI Centre for Doctoral Training in Transformative Pharmaceutical Technologies. My PhD project focuses on developing immunomodulatory alginate analogues for mitigating the foreign body reaction (FBR), an immune response that can jeopardize functionalities of implants and their therapeutic outcomes. As part of my PhD training program, I also carried out a 3-month training project on the development and application of fluorescent nanosensors for real-time measurements of pH in complex model systems, and an industry placement at Nemaura Pharma. |
Consuelo Coser, PhD Student at University of Nottingham |
References
1 CHAPLIN, D. D. M. D. P. Overview of the immune response. Journal of allergy and clinical immunology, NEW YORK, v. 125, n. 2, p. S3-S23, 2010. ISSN 0091-6749.
2 MARSHALL, J. S. et al. An introduction to immunology and immunopathology. Allergy, asthma, and clinical immunology, England, v. 14, n. Suppl 2, p. 49-49, 2018. ISSN 1710-14841710-1492.
3 NOSKOVICOVA, N.; HINZ, B.; PAKSHIR, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells (Basel, Switzerland), BASEL, v. 10, n. 7, p. 1794, 2021. ISSN 2073-4409.
4 HIRAYAMA, D.; IIDA, T.; NAKASE, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. International journal of molecular sciences, BASEL, v. 19, n. 1, p. 92, 2018. ISSN 1661-65961422-0067.
5 HUANG, X. et al. Polarizing Macrophages In Vitro. Macrophages, New York, NY, v. 1784, p. 119-126, 2018. ISSN 1064-3745.
6 SHEIKH, Z. et al. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials, BASEL, v. 8, n. 9, p. 5671-5701, 2015. ISSN 1996-1944.
7 ROSZER, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators of Inflammation, LONDON, v. 2015, p. 816460-16, 2015. ISSN 0962-93511466-1861.
8 KRZYSZCZYK, P. et al. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Frontiers in physiology, LAUSANNE, v. 9, n. MAY, p. 419-419, 2018. ISSN 1664-042X.
9 ABARICIA, J. O. et al. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta biomaterialia, England, v. 133, p. 58-73, 2021. ISSN 1742-7061.
10 VASSEY, M. J. et al. Immune Modulation by Design: Using Topography to Control Human Monocyte Attachment and Macrophage Differentiation. Advanced science, Weinheim, v. 7, n. 11, p. 1903392-n/a, 2020. ISSN 2198-3844.
Related Content
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.


