Immune Response
Immune Response: markers and related antibodies
Introduction
The human immune system contains a collection of different cell types and molecules that help to protect the body from toxins, viral infections, bacteria, and parasites. Although immunology covers all aspects of this complex network, the field is united by the immune cell. The central points of immunology are: understanding, identifying, and distinguishing the many different immune cells that are essential for diagnosis and therapy. This includes research on both the innate and adaptive immune response and processes such as pathogen recognition, immune cell activation and recruitment, cytokine secretion, inflammation, and antibody production. Cell markers are helpful tools for immunophenotyping diverse types of human immune cells. This page offers a detailed choice of the most commonly used cell markers and a small selection of antibodies used in immunology.
 |
|
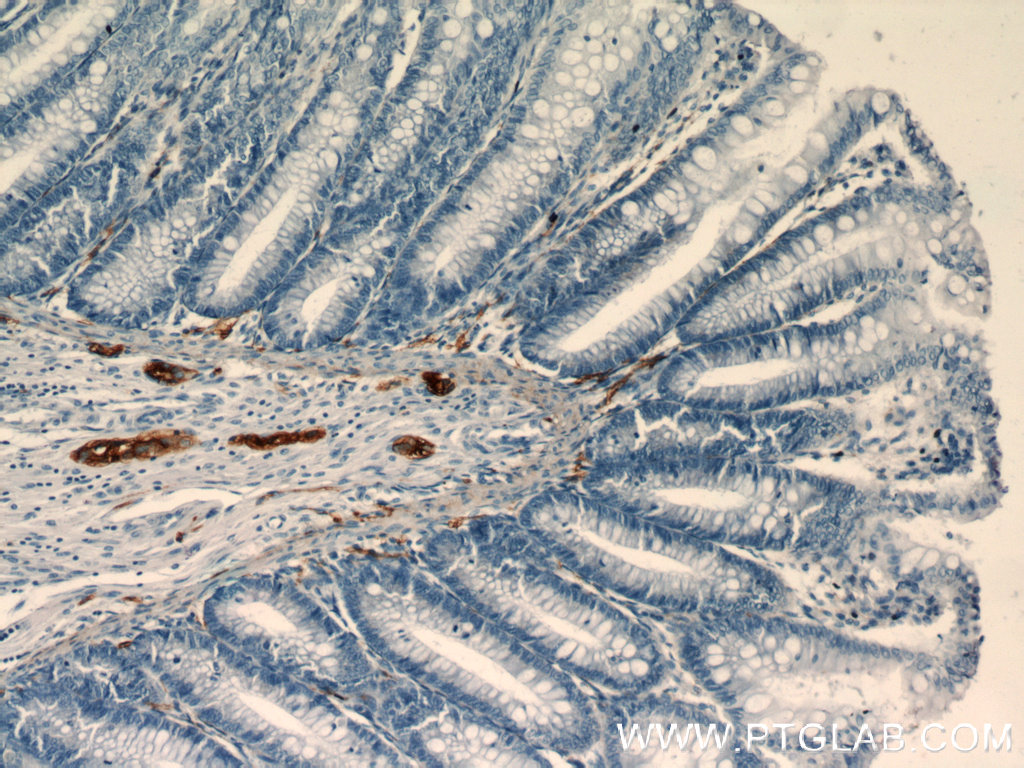
Immunohistochemical staining of paraffin-embedded human colon slide using NCAM1 antibody (14255-1-AP) at a dilution of 1:400. |
Natural killer (NK) cells and NCAM1/CD56
Natural killer (NK) cells are the most responsive immune cells to acute exercise. Their sensitivity to physiological stress combined with their role in innate immune defenses shows that there is a link between regular physical activity and overall health status. NK cells are a heterogeneous population consisting of at least two distinct subsets based on the expression intensity of neural cell adhesion molecule 1 (NCAM1, also known as CD56).
NCAM1/CD56 is a cell adhesion molecule involved in neuron-neuron adhesion, neurite fasciculation, an outgrowth of neurites, synaptic plasticity, neurodevelopment, and neurogenesis. Besides NK cells, NCAM1 is also expressed on the surface of human neurons, glial cells, and skeletal muscle cells. It is also involved in the expansion of CD4+ and CD8+ T cells and dendritic cells, which play an important role in immune surveillance. There are three main forms of human NCAM-1 that arise by alternative splicing: 120kDa (761 amino acids (aa) (glycosylphosphatidylinositol (GPI) anchored)), 140kDa (848 aa, short cytoplasmic domain), and 180kDa (1120 aa, long cytoplasmic domain).
NCAM1/CD56 is already broadly used as a sensitive and diagnostically useful immunohistochemical marker for problematical small cell lung carcinomas or neuroendocrine tumors
 |
|
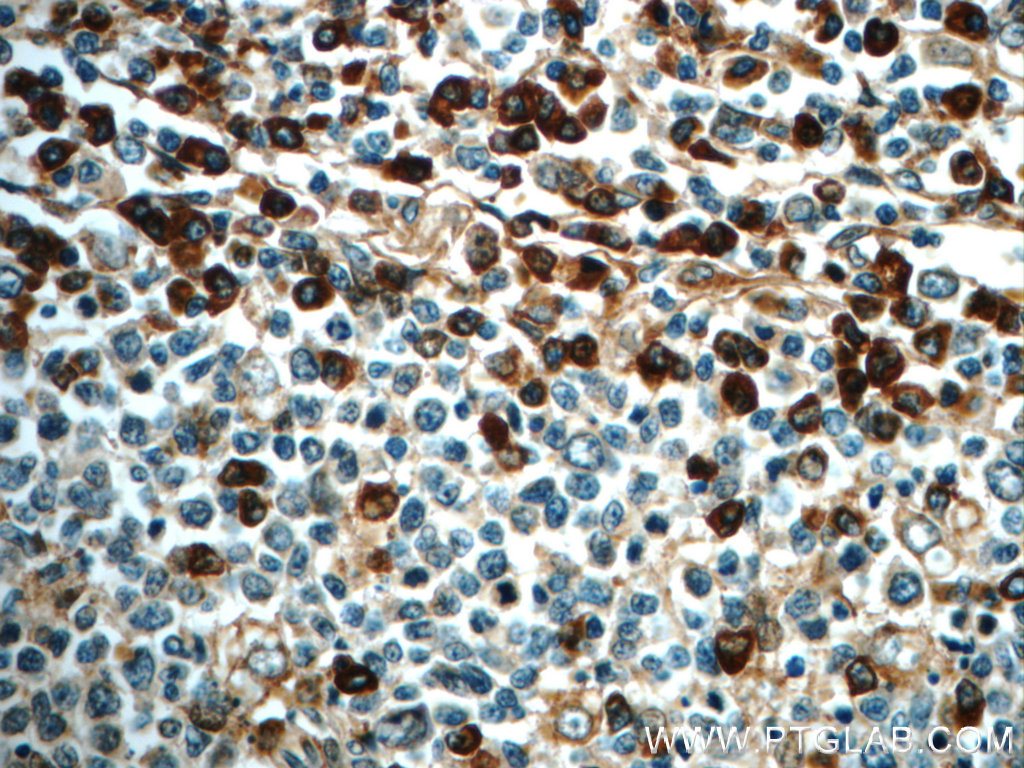
IHC staining of paraffin-embedded human tonsillitis slide using IL6 antibody (21865-1-AP) at a dilution of 1:50. |
A keystone cytokine – the many faces of Interleukin-6
Interleukin-6 (IL-6) acts as both a proinflammatory and an anti-inflammatory cytokine. It is an endogenous chemical, active in inflammation and in B cell maturation.
In the early phase of infectious inflammation, IL-6 is produced by monocytes and macrophages immediately after the stimulation of Toll-like receptors (TLRs) with distinct pathogen-associated molecular patterns (PAMPs). In non-infectious inflammations, such as a burn or traumatic injury, damage-associated molecular patterns (DAMPs) from damaged or dying cells stimulate TLRs to produce IL-6. This acute IL-6 expression plays a significant role in host defense by stimulating various cell populations. IL-6 is released by monocytes and macrophages in response to other inflammatory cytokines, which include interleukin-11 and tumor necrosis factor (TNF)-beta.
IL-6 is a molecule with multiple forms and functions depending on where it is secreted. IL-6 helps T cells to differentiate in their development. It is required for progenitor cell development and helps B cells differentiate and proliferate, and it promotes the formation of plasma cells from B cells. IL-6 also plays a vital role in the development of blood cells, whether white cells, red cells, or platelets. IL-6 also leads to the activation of osteoclasts and osteoporosis. It also induces the secretion of vascular endothelium growth factor (VEGF), which leads to the increased growth of blood vessels and vascular permeability in inflammation.
Genetic variations of IL6 are associated with rheumatoid arthritis systemic juvenile (RASJ), and polymorphism of IL6 promoter can be an indicator for Kaposi sarcoma development in HIV-infected men.
 |
|
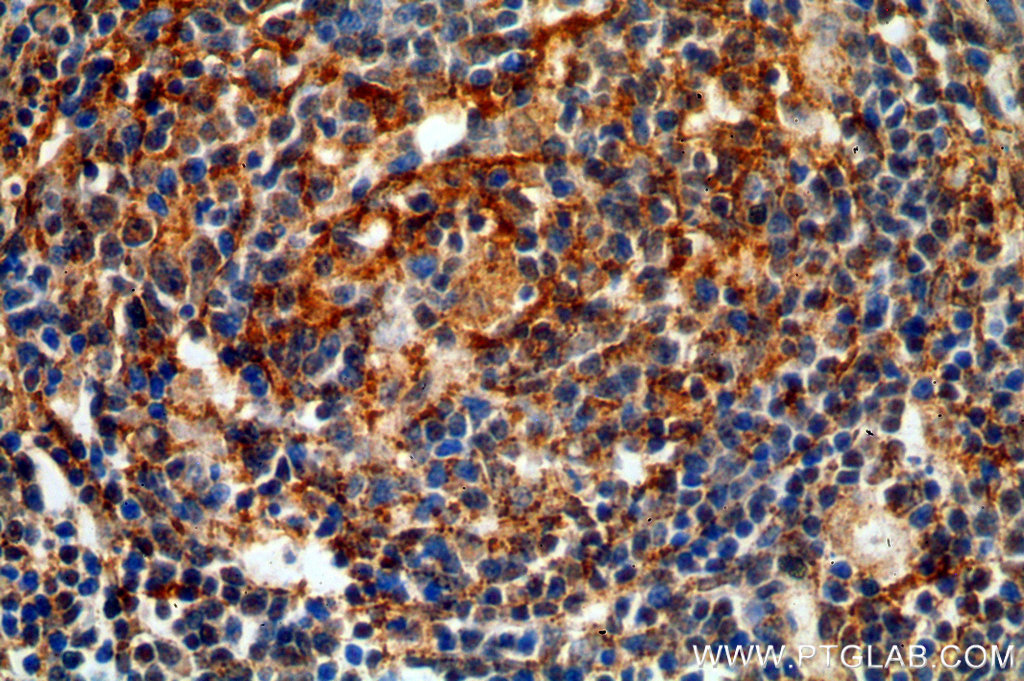
Immunohistochemical of paraffin-embedded human spleen using 19272-1-AP (TNFR2 antibody) at dilution of 1:50 (under 40x lens) |
Multiple roles for Tumor Necrosis Factor-Alpha (TNFA/TNFSF2)
Tumor necrosis factor-alpha (TNFA/TNFSF2) is an important multifunctional cytokine that regulates inflammation, host defense, immune responses, and apoptosis. TNFA/ TNFSF2 signals through two distinct cell surface receptors, TNFR1 (TNFRSF1A, CD120a, p55) and TNFR2 (TNFRSF1B, CD120b, p75). TNFR1 is widely expressed, whereas TNFR2 exhibits more restricted expression, being found on CD4 and CD8 T lymphocytes, endothelial cells, microglia, oligodendrocytes, neuron subtypes, cardiac myocytes, thymocytes, and human mesenchymal stem cells. TNFA/TNFSF2 is mainly secreted by macrophages.
The main biologic function is to induce inflammation via the upregulation of gene transcription, primarily through the NFkB and AP-1 signaling pathways, which leads to the expression of a large number of genes. TNF plays a significant role in innate immunity and host defense against bacterial, fungal, and parasitic pathogens and is particularly important for host defense against intracellular organisms such as Mycobacterium tuberculosis. Dysregulation of TNFA/TNFSF2 has been found to be involved in tumorigenesis, tumor metastasis, viral replication, septic shock, fever, inflammation, autoimmune diseases including Crohn’s disease, in addition to rheumatoid arthritis and graft-versus-host disease.
Recently, growing evidence has shown that a blocking of TNFA/TNFSF2 action in patients and experimental animal models increases disease activity and severity in some T-cell dependent autoimmune diseases (e.g., multiple sclerosis or Type 1 diabetes).