Are Mesenchymal Stem Cells (MSCs) true stem cells?
A guide to MSC characteristics, differentiation pathways and supporting reagents for MSC culture.
MSCs have great potential and clinical significance for tissue engineering. They are adult multipotent cells capable of differentiating to different types of tissues.
1) What are mesenchymal stem cells (MSCs)?
MSCs are multipotent stem cells. Below are a few facts about MSCs (1):
- Discovered by Alexander Friedenstain in the 1960s–70s
- They are multipotent cells found principally in bone marrow
- Their main function is to make and repair skeletal tissues such as cartilage and bones
- They are defined as a plastic-adherent cell population
- They are considered to be the most promising tools for cell and cell-based gene therapy.
Similar to Hematopoietic Stem Cells (HSCs), MSCs are also found in bone marrow (present at 0.001–0.01%) (1). MSCs are commonly known as “multi-lineage cells” because of their wide range of potential applications. They are known to regenerate structure and connective tissues, repair bones(2) and cartilage(3), stimulate angiogenesis(4) (after heart attacks), and reduce inflammation and scarring through their immunomodulatory properties(5). They can also be found in adipose tissue, amnion, synovial fluids, muscles, dermis, deciduous teeth, and umbilical cord tissue. The number of cells obtained from a donor is insufficient to be able to produce a fully matured organ. Because of this, MSCs need to be grown and expanded in vivo with controlled media and specific growth factors in order to undergo senescence after 8–10 passages(1,6). MSCs are multipotent stem cells that are able to differentiate into a variety of different cells. The four main types are adipocytes, myocytes, chondrocytes, and osteocytes (Figure 1). MSCs are long, thin, and widely dispersed. They have a great capacity for self-renewal compared to other stem cells and also possess an immunomodulatory capacity that has clinical potential in immune diseases such as Graft versus Host Disease (GVHD), aplastic anemia (AA), Crohn’s disease (CD), rheumatoid arthritis (RA), and multiple sclerosis (MS) (13).
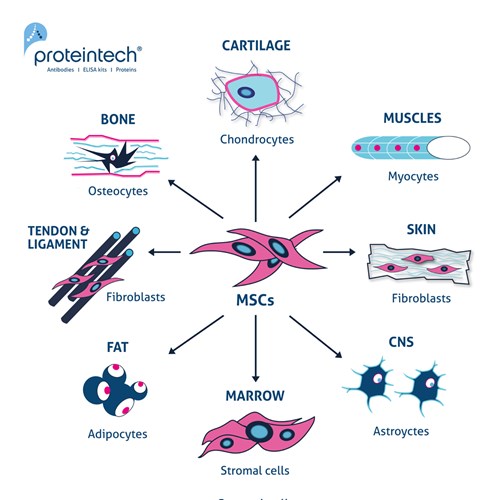
Figure 1. The multipotent potential of MSCs. Exposure to different inductive agents (e.g., cytokines and growth factors) can differentiate MSCs into chondrocytes, myocytes, fibroblasts, astrocytes, stromal cells, adipocytes, and osteocytes.
2) Mesenchymal Stem Cells: is it time to change the name?
Commonly, MSCs are thought to be true stem cells as they can undergo self-renewal and differentiation into a plethora of tissues of the mesenchymal lineage (6). However, MSCs are a particular type of pre-differentiated stem cells. They remain undifferentiated, but they are not true stem cells. Figure 2 contains further detail on the differentiation pathways of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs and iPCs differentiate into Ectoderm, Mesoderm, and Endoderm lineages.
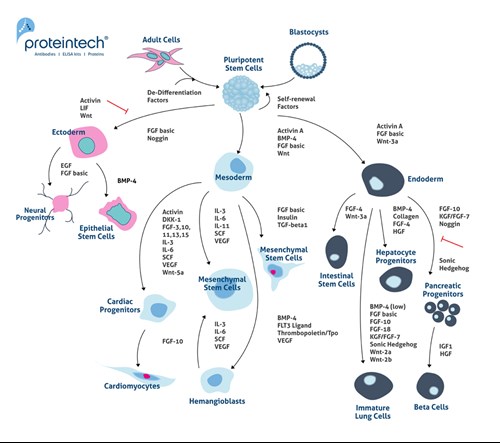
Figure 2. Differentiation pathways for embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs and iPCs differentiate into ectoderm, mesoderm, and endoderm lineages.
3) Which growth factors and cytokines are necessary for mesenchymal stem cells (MSCs) differentiation?
Depending on the environment MSCs grow in, they can give rise to a variety of lineage-specific cell types. The four principal cell types they differentiate into are myocytes, chondrocytes, osteocytes, and adipocytes. Table 1 contains a summary of the key growth factors needed for the differentiation process.
|
Cell type |
Growth Factor Used for Differentiation |
|
Myocytes |
|
|
Chondrocytes |
|
|
Osteocytes |
|
|
Adipocytes |
Table 1. Growth factors and cytokines needed for MSCs differentiation into a specific cell type.
4) How do mesenchymal stem cells (MSCs) support tissue regeneration?
Bone
The potential of MSCs for tissue repair and wound healing is well known. It has been shown extensively in the literature that MSCs and their progenitors are able to differentiate into chondrocytes under certain conditions in vivo and in vitro (2). Furthermore, these cells are nonimmunogenic; thus, allogeneic transplant of MSCs does not require host immunosuppression (7). Over the years, several experiments have shown that the implantation of human bone marrow-derived MSCs at fracture sites has resulted in bone repair in animal models (7,8). Furthermore, human non-union fractures that lead to morbidity, prolonged hospitalization, and increased expenses have also been repaired with percutaneous bone marrow grafting (7,9).
Cartilage
MSCs are able to repair cartilage. Indeed, depending on the culture environment, MSCs can differentiate into chondrocytes and stimulate chondrogenesis (3). It has been identified that a constant supply of insulin-like growth factor-1 (IGF-1) and transforming growth factor-β1 (TGF-β1), alone and in combination, regulate the proliferation and differentiation of periosteal mesenchymal cells during chondrogenesis (3).
Heart tissue
Cardiovascular disease is the second biggest cause of death worldwide after cancer. Outside of lifestyle interventions and preventative measures, there is a critical need to discover sound therapies. MSCs affect post-acute myocardial infarction remodeling, stimulate regeneration of injured cardiac tissue, and induce coronary artery angiogenesis (10). Furthermore, MSCs can also differentiate into cardiac cell types, although complete differentiation into functional cells has not yet been achieved. This could be used as an alternative strategy for augmenting the function of diseased myocardium and ultimately be used for cellular cardiomyoplasty (11).
5) What are the future prospects for mesenchymal stem cells (MSCs)?
MSCs still require approval by the Food and Drug Administration (FDA). Currently, this process is delayed by inconsistent immunosuppression results, variability in cell quality, inconsistent protocols, varying dosages, and differing transfusion patterns (12).
One of the current challenges is avoiding the differentiation of MSCs into lineage-specific mature cells (12). In order to better understand the mechanism behind differentiation events, the well-known growth factors (e.g., FGF basic HZ-1285, FGF-4 HZ-1218, IL-6 HZ-1019, PDGF-bb HZ-1308) and their interactions with MSCs need to be investigated further. It is only when the regulatory checkpoints have been attained that the full potential of MSCs can be exploited for diverse cellular therapies in humans.
References
- Mesenchymal stem cells: clinical applications and biological characterization.
- Multilineage mesenchymal differentiation potential of human trabecular bone‐derived cells.
- Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro.
- Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application.
- Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives.
- Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines.
- Mesenchymal Stem Cells for Bone Repair and Metabolic Bone Diseases
- Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells.
- Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells.
- Mesenchymal Stem Cells
- Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart
- Mesenchymal stem cells and immunomodulation: current status and future prospects
- Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases