PLSCR1: An exciting new defender against COVID-19
Written by Sophie Williams; Biochemistry PhD researcher at the University of Bristol.
Summary of the paper: Xu, D., Jiang, W., Wu, L. et al. PLSCR1 is a cell-autonomous defence factor against SARS-CoV-2 infection. Nature (2023). https://rdcu.be/dpKNo
Cell-autonomous immunity describes self-defense mechanisms that occur within cells to combat insults, including viral infections. These immunity pathways are utilized broadly by bacteria, plants, and animal cells, and in humans can provide the individual cell with a safeguard against pathogens including HIV-1 and Mycobacterium tuberculosis.
The role of cell-autonomous immunity pathways in combating SARS-CoV-2 infection is not currently understood. The type II cytokine IFNγ is known to be a fundamental orchestrator in cell-autonomous immunity pathways in most nucleated cells, while the increased production of IFNγ has also been linked to enhanced protection against COVID-19 in young adults and children. Additionally, genetic lesions in the IFN signaling pathway have been associated with severe COVID-19 disease, highlighting a role for cell-autonomous immunity in protection against SARS-CoV-2.
Despite this, the involvement of cell-autonomous immunity in combating SARS-CoV-2 infection has not been explored, with most of the current therapeutic research focused on the development of neutralizing antibodies. Yet, IFNγ may act as a key defender against SARS-CoV-2 infection, and further understanding may aid in the development of future therapeutics.
In the article “PLSCR1 is a cell-autonomous defence factor against SARS-CoV-2 infection”, the authors uncover a factor that acts as a first line of defense against virulent coronaviruses. Xu et al., used six Proteintech products in their study, including the antibody targeting the titular protein PLSCR1 (11582-1-AP).
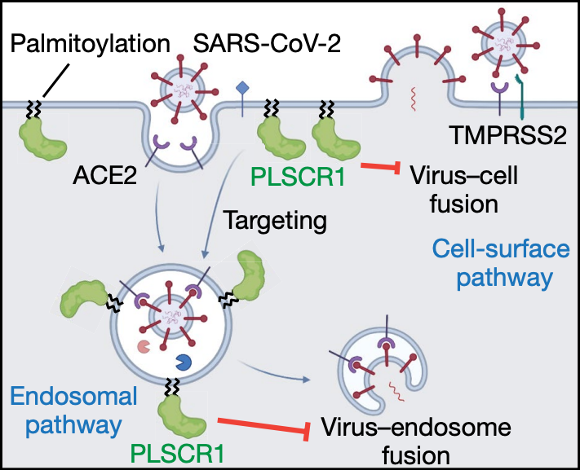
Figure 1. PLSCR1 restricts SARS-CoV-2 entry via multiple virus-cell fusion pathways (figure taken from Xu et al., 2023).
Human PLSCR1 inhibits SARS-CoV-2
As IFNγ mutations have been linked to severe COVID-19 disease and IFNγ is a known key component in cell-autonomous immunity pathways, Xu and the team began by investigating the ability of recombinant IFNγ to restrict SARS-CoV-2 infection in two separate cell lines: Huh7.5 hepatoma cells and human lung epithelial A549 cells (which mimic host-cell targets within the respiratory tract). This further confirmed that IFNγ does indeed inhibit SARS-CoV-2 infection. However, as IFNγ is involved in larger signaling pathways, the question of which IFNγ-induced protein conferred this enhanced resistance was raised.
Parallel genome-wide loss-of-function screens were applied to determine which of the 19,050 tested genes conferred restriction to SARS-CoV-2 infection in the presence of IFNγ. Using a fluorescently labeled SARS-CoV-2 construct, fluorescence-activated cell sorting (FACS), and next-generation sequencing, key genes involved in host defense against SARS-CoV-2 were identified. Within this group of defensive factors, genes involved in the IFNγ signaling pathway that had previously been identified as SARS-CoV-2 restrictors were identified. Interestingly, a phospholipase scramblase (PLSCR1) was also reported to have antiviral activities in the screen; however, its influence over SARS-CoV-2 infection was uncharacterized.
PLSCR1 mRNA is known to be naturally upregulated in the upper respiratory tract of people infected with SARS-CoV-2, while PLSCR1 protein expression is strongly induced by IFNγ in human primary tracheal epithelia. Xu and colleagues went on to show that PLSCR1 loss in lung epithelial cells led to a 4.5-fold increase in viral load when treated with SARS-CoV-2.
How does PLSCR1 restrict SARS-CoV-2 infection?
At this point in the study, Xu and colleagues confirmed that PLSCR1 provides host defense against SARS-CoV-2 infection; however, the next big question concerned the mechanism behind this resistance. At which stage of the viral life cycle is PLSCR1 acting against infection? To address these questions, cells were infected with replication incompetent HIV-1 pseudotype viruses encoding SARS-CoV-2 spike proteins from a range of strains. The authors showed that loss of PLSCR1 enhanced the ability of the pseudovirus to infect cells, suggesting PLSCR1 may act in blocking the entry of beta-coronaviruses into the host cell.
To further delve into the ability of PLSCR1 to inhibit SARS-CoV-2 entry, a combination of single-molecule nanoscopy and the bipartite NanoLuc reporter assay was applied. SARS-CoV-2 has previously been shown to invade host cells via two routes: the endocytic route or via TMPRSS2-dependent fusion with the host’s plasma membrane. The authors provided evidence that PLSCR1 disrupted SARS-CoV-2 uptake via both pathways. It was shown that PLSCR1 directly targets SARS-CoV-2 containing vesicles to prevent fusion with the host cell membranes, therefore halting viral escape into the host’s cytosol.
Finally, the structural basis of PLSCR1 activity was investigated. PLSCR1 sequence analysis identified the presence of a palmitoylation motif, the mutation of which resulted in disrupted localization of PLSCR1 to the plasma membrane and the abolishment of PLSCR1’s anti-SARS-CoV-2 activity. The presence of a 12-stranded β-barrel within PLSCR1 was predicted via structural modeling, and through complement assays it was highlighted that the first strand within the β-barrel was essential for antiviral effect. The study contains evidence that PLSCR1’s β-barrel domain is vital in preventing the fusion of the virus with the host cell once it is docked onto the target membrane via palmitoylation, providing the first mechanistic insight into this anti-COVID factor. The importance of PLSCR1’s β-barrel in anti-COVID activity has previously been reported, with a single nucleotide polymorphism in the human PLSCR1 locus – affecting a residue within the PLSCR1 β-barrel – being linked with susceptibility to severe COVID symptoms.
Implications and future directions
The findings presented in this study provide a mechanistic insight into a novel SARS-CoV-2 restrictor: PLSCR1. The study provides evidence that PLSCR1 acts to block viral coronavirus entry into host cells via multiple entry pathways and is functionally conserved across mammalian evolution. These findings provide a further understanding of how the innate immune system acts as a first line of defense against invading SARS-CoV-2 infection. Overall, this study highlights the need to further understand how cell-autonomous immunity provides protection against viral infection. This will not only expand our current knowledge of virus pathogenesis but will also give us the best grounds to develop novel therapeutics.
Proteintech products used in this publication:
- PLSCR1 polyclonal antibody (11582-1-AP)
- GAPDH monoclonal antibody (60004-1-Ig)
- GFP tag monoclonal antibody (66002-1-Ig)
- ChromoTek Halo monoclonal antibody (28a8)
- TMEM41B polyclonal antibody (29270-1-AP)
- IFITM3 polyclonal antibody (11714-1-AP)
Are you researching COVID-19? Proteintech sells a wide range of top-cited antibodies and ELISA kits to study the SARS-CoV-2 virus and immune response.
Related Content
Immune Response Featured Products
The multi-organ impact of COVID-19
Building research careers in a post-COVID world
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

