The multi-organ impact of COVID-19
Written by Alicia Haydo M.Sc., a PhD candidate at Goethe University Frankfurt am Main
COVID-19 is a respiratory disease caused by infection with the SARS-CoV-2 virus. The virus originated in Wuhan, China, from where it spread and caused a global pandemic, and after almost 3 years, the virus continues to infect people all over the world. COVID-19 is best known for its well-documented respiratory effects; however, it does not only affect the lungs (Jain 2020). During infection and after recovery, COVID-19 has many detrimental effects on organs of the human body, with many longer-term issues yet to be elucidated.
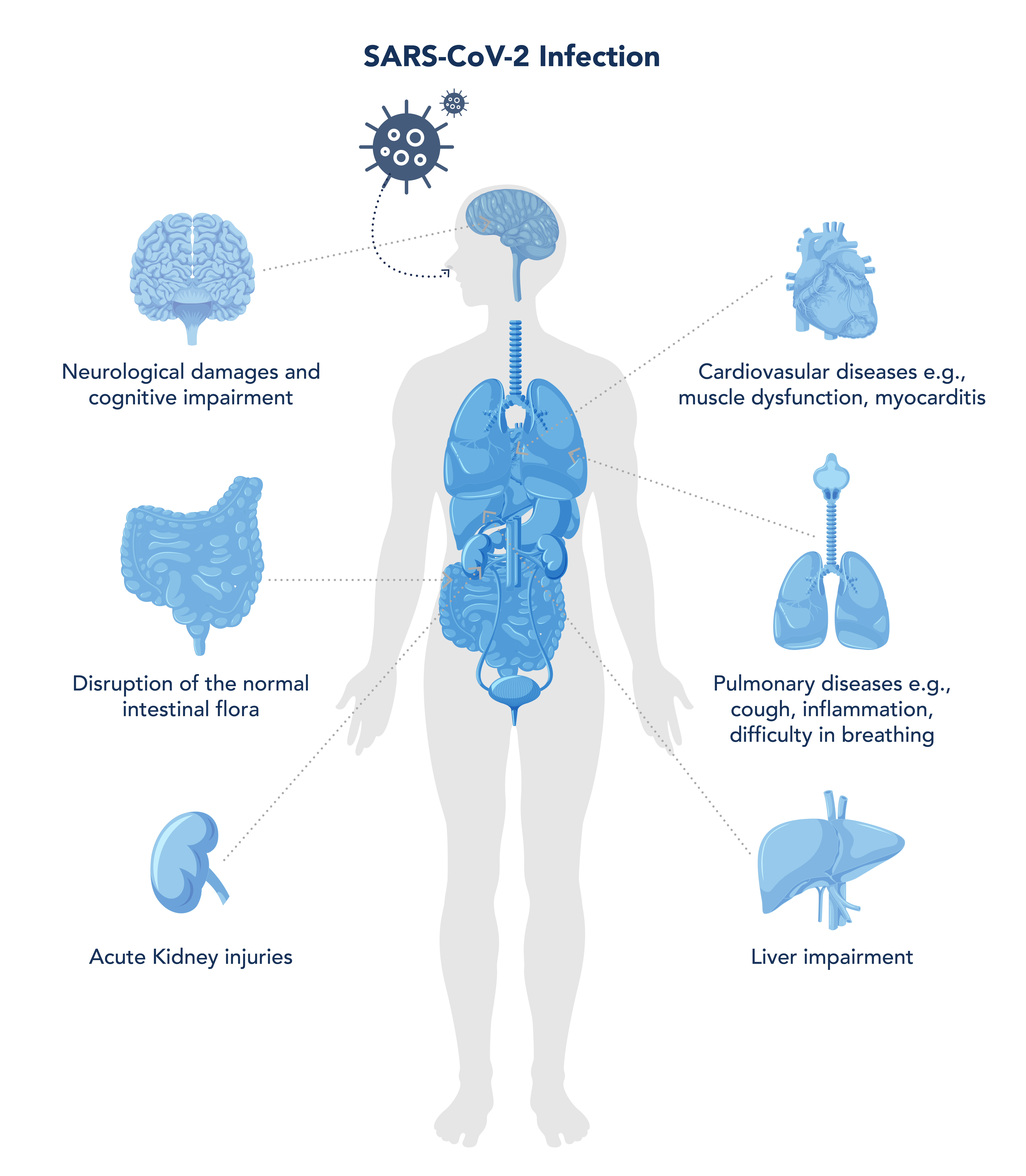
Figure 1. Graphic summarizing COVID-19 effects on different organs. Property of Proteintech Group.
1. Effects of COVID-19 on the heart
Cardiovascular disease is one of the most common comorbidities alongside COVID-19 infection, although the specific mechanisms of myocardial damage following infection remain unclear. It is posited that the virus can damage the heart in two ways: a.) it can directly enter the myocardial tissue and cause damage via ACE2 receptors, which are widely distributed in the cardiovascular system, or b.) it can initiate an uncontrolled cytokine storm leading to systemic damage. Increased mortality may depend on an individual’s underlying risk of cardiovascular disease, which in turn elevates the risk of heart abnormalities following COVID-19 infection (Shah et al. 2021). It is reported that the virus can directly infect vascular endothelial cells, causing apoptosis and altered interaction with platelets and leukocytes in circulation. Furthermore, the imbalanced endothelial release of vasoactive substances including nitric oxide and prostaglandins, and increases in reactive oxidative species, lead to the “immunothrombosis” that is thought to contribute to many of the multi-system manifestations of COVID-19 (Roy et al. 2022). The detrimental consequences are thought to be cardiac muscle dysfunction presenting as heart failure, myocarditis, cell necrosis, and arrhythmias (Shah et al. 2021).
2. Effects of COVID-19 on the liver
Another non-pulmonary comorbidity after a SARS-CoV-2 infection is liver impairment. The levels of liver-associated enzymes, e.g., alanin aminotransferase or aspartate aminotransferase, alter to various degrees in COVID-19 patients. It is presumed that the virus may directly cause liver impairment, since ACE2 receptors are present in hepatocytes and cholangiocytes in the liver (Shah et al. 2021). In one study, patients hospitalized with COVID-19 had a >75% chance of abnormal liver tests, with around 20% of patients also acquiring a liver injury (Cai et al. 2020). Lastly, COVID-19 has been linked to an overt proinflammatory cytokine profile, which probably contributes substantially to the observed early and late liver abnormalities (Dufour et al. 2022).
3. Effects of COVID-19 on the gastrointestinal tract
Much evidence suggests that SARS-CoV-2 affects the digestive system of infected patients, as shown in various symptoms such as diarrhea, loss of appetite, nausea, vomiting, and abdominal pain. These gastrointestinal effects of COVID-19 present major challenges for patients in achieving their nutritional needs. Due to a lack of nutrient contact with the intestinal mucosa, a deterioration of immune system functions can occur, resulting in an increase in bacterial translocation. It is proposed that these effects of SARS-CoV-2 on the gastrointestinal tract are due in part to the high abundance of the ACE2 receptor in the ileum and colon. Further, ACE2 is highly expressed in the human small intestine, especially in proximal and distal enterocytes, which are directly exposed to foreign pathogens and food particles. It is believed that the virus attaches to the ACE2 receptors of the digestive system and disrupts the normal intestinal flora, resulting in various gastrointestinal symptoms (Shah et al. 2021). In addition, Meringer et al. (2022) showed evidence of persistent and aberrant inflammation as well as induction of autoimmunity in a subset of patients with post-acute COVID-19 syndrome.
4. Effects on the brain
Smell dysfunction, muscle pain, headaches, encephalopathy, dizziness, and dysgeusia are common neural comorbidities from a mild case of SARS-CoV-2 infection. In severe cases, it is accompanied by seizures, movement disorders, motor and sensory deficits, ataxia, and strokes. In a handful of patients, these symptoms can remain even after a full recovery from the initial infection. The SARS-CoV-2 virus is thought to enter the brain via the bloodstream, where it attaches to the endothelium via ACE2 receptors and thus alters the blood-brain barrier, enabling the virus to spread in the central nervous system. In addition, SARS-CoV-2-associated cytokines such as interleukin (IL) 1b, IL-17, IL-6, and tumor necrosis factor alpha (TNFa) can alter the permeability of the blood-brain barrier, thus allowing the virus to enter (Shah et al. 2021). In particular, the olfactory region plays a critical role in the neuroinvasion of SARS-CoV-2. Loss of smell and taste is a well-documented symptom of infection that results from cell death following the virus hijacking olfactory neurons, which then act as carriers for the virus from the respiratory tract to the brain (Shah et al. 2021).
5. Effects on the kidney
Lastly, acute kidney injuries are another potential comorbidity of COVID-19 infection and can be associated with increased mortality. The mechanism of action for kidney injury relates to expression of the ACE2 gene, which is highly expressed in the kidneys and bladder. This indicates that the urinary system can act as another potential route by which the virus infects the body, along with the respiratory, hepatic, and digestion systems (Shah et al. 2021). In addition, the SARS-CoV-2 virus can trigger acute tubular necrosis. Moreover, in some cases, direct viral tropism of the kidneys and collapsing focal segmental glomerulosclerosis have been observed (Liakopoulos et al. 2022). It is also possible for the medicine prescribed to treat COVID-19 to indirectly affect the kidney, e.g., remdesivir, which has been associated with an increased chance of reporting renal and urinary disorders in patients (Silva et al. 2021).
Future research
Overall, scientists are interested in developing treatments for acute COVID-19, as well as long COVID-19 cases. An interesting approach by Cau et al. (2022) details AI-based models, which can be applied in patients with long COVID-19 to assist clinicians and reduce the considerable impact on the care and rehabilitation unit. Long COVID in particular leads to widespread systemic dysfunctions, meaning various therapeutic strategies have to be explored to help combat this multi-factorial disorder. Anti-inflammatory treatments are a key area of research for long COVID treatments in order to combat the extensive inflammatory state from infection. Popular options include antibody treatments such as Infliximab, Tocilizumab, Siltuximab, Anakinra, and Leronlimab. Antidepressants such as Vortioxetine, a selective serotonin reuptake inhibitor and serotonin receptor modulator, are being investigated to treat long COVID (Koc et al. 2022). Lastly, scientists are trying to understand the mechanisms of action of the virus by using model systems, e.g., organoids. Kim et al. (2022), in their summary, documented that organoids revealed cellular tropism of the virus in different organs and identified potential drug candidates that could help treat the disease.
Conclusion
Emerging evidence on the pathway of entry and distribution of the SARS-CoV-2 virus in organs suggests the ability of the virus to effectively replicate in the lungs, heart, liver, gastrointestinal tract, brain, and kidneys. The development of experimental models, e.g., organoids and other novel methods to test further hypotheses will lead the way for future research to finally decipher and treat the virus.
Proteintech SARS-CoV-2 ELISA Kits
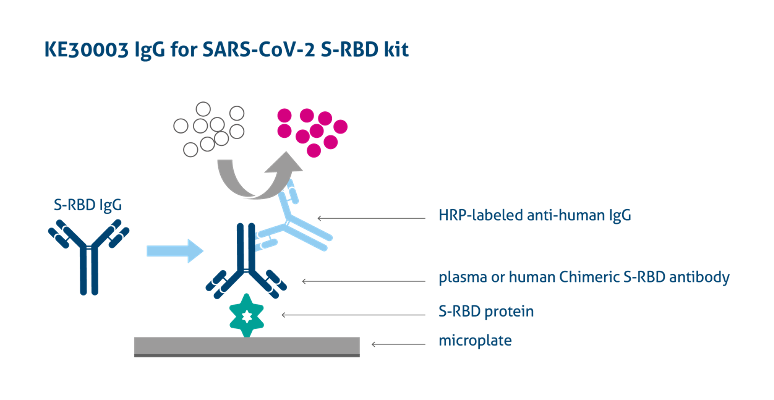
Image from: https://www.ptglab.com/products/Virus-IgG-for-2019-nCoV-S-RBD-ELISA-Kit-KE30003.htm
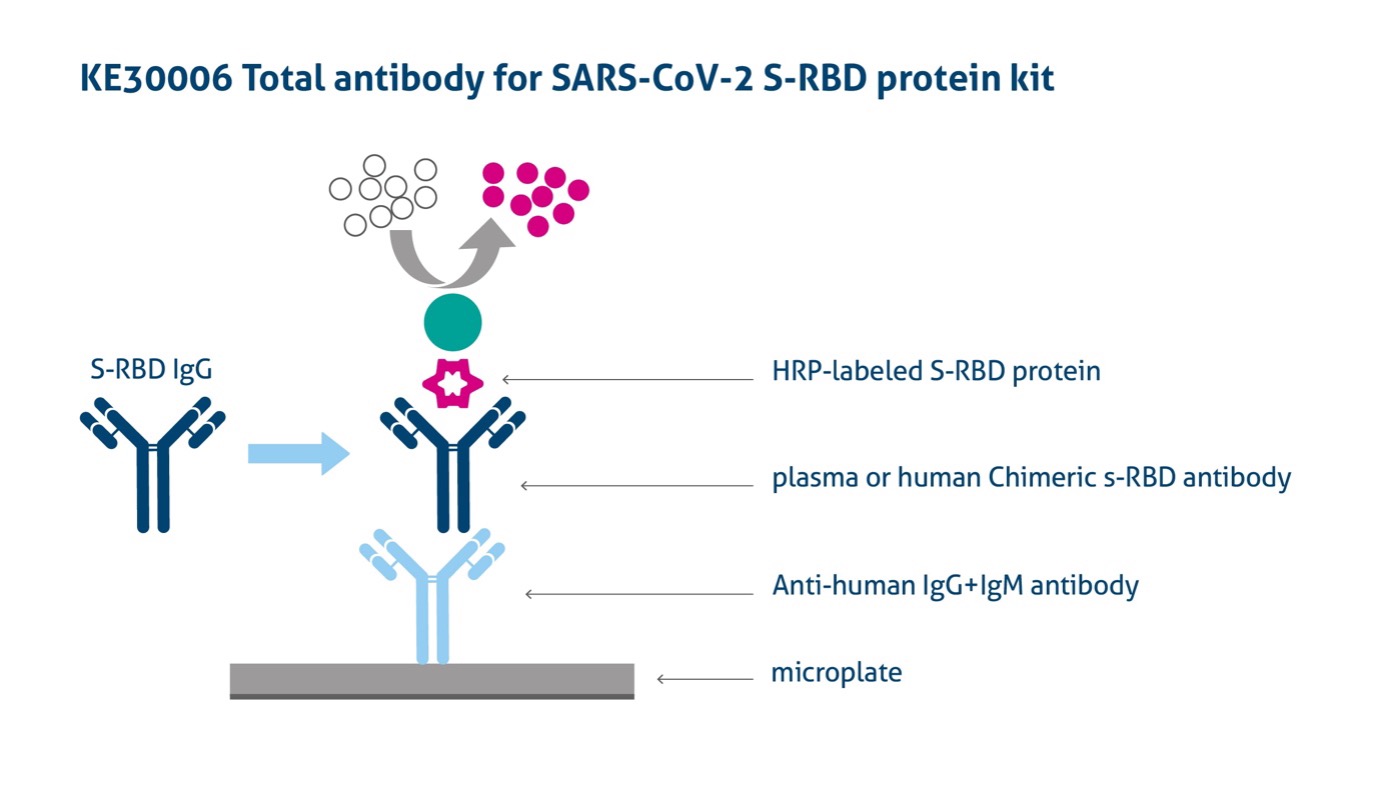
Image from: https://www.ptglab.com/products/Virus-IgG-for-2019-nCoV-S-RBD-ELISA-Kit-KE30003.htm
Visit our dedicated page to find all our reagents for COVID-19 research: Click here.
Best-selling COVID-19 ELISA kits: Click here.
References
- Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020 Sep;73(3):566-574. doi: 10.1016/j.jhep.2020.04.006. Epub 2020 Apr 13. PMID: 32298767; PMCID: PMC7194951.
- Cau R, Faa G, Nardi V, Balestrieri A, Puig J, Suri JS, SanFilippo R, Saba L. Long-COVID diagnosis: From diagnostic to advanced AI-driven models. Eur J Radiol. 2022 Mar;148:110164. doi: 10.1016/j.ejrad.2022.110164. Epub 2022 Jan 19. PMID: 35114535; PMCID: PMC8791239.
- Dufour JF, Marjot T, Becchetti C, Tilg H. COVID-19 and liver disease. Gut. 2022 Nov;71(11):2350-2362. doi: 10.1136/gutjnl-2021-326792. Epub 2022 Jun 14. PMID: 35701093.
- Esposito F, Cirillo M, De Micco R, Caiazzo G, Siciliano M, Russo AG, Monari C, Coppola N, Tedeschi G, Tessitore A. Olfactory loss and brain connectivity after COVID-19. Hum Brain Mapp. 2022 Apr 1;43(5):1548-1560. doi: 10.1002/hbm.25741. Epub 2022 Jan 27. PMID: 35083823; PMCID: PMC8886650.
- Jain U. Effect of COVID-19 on the Organs. Cureus. 2020 Aug 3;12(8):e9540. doi: 10.7759/cureus.9540. PMID: 32905500; PMCID: PMC7470660.
- Kim J, Koo BK, Clevers H. Organoid Studies in COVID-19 Research. Int J Stem Cells. 2022 Feb 28;15(1):3-13. doi: 10.15283/ijsc21251. PMID: 35220288; PMCID: PMC8889327.
- Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its Management. Int J Biol Sci. 2022 Jul 11;18(12):4768-4780. doi: 10.7150/ijbs.75056. PMID: 35874958; PMCID: PMC9305273.
- Liakopoulos V, Roumeliotis S, Papachristou S, Papanas N. COVID-19 and the kidney: time to take a closer look. Int Urol Nephrol. 2022 May;54(5):1053-1057. doi: 10.1007/s11255-021-02976-7. Epub 2021 Aug 12. PMID: 34383205; PMCID: PMC8358250.
- Meringer H, Mehandru S. Gastrointestinal post-acute COVID-19 syndrome. Nat Rev Gastroenterol Hepatol. 2022 Jun;19(6):345-346. doi: 10.1038/s41575-022-00611-z. PMID: 35383321; PMCID: PMC8981882.
- Muhammad Dawood Shah, Aini Simon Sumeh, Muhammad Sheraz, Muthu Subash Kavitha, Balu Alagar Venmathi Maran, Kenneth Francis Rodrigues, A mini-review on the impact of COVID 19 on vital organs, Biomedicine & Pharmacotherapy, Volume 143, 2021, 112158, ISSN 0753-3322, https://doi.org/10.1016/j.biopha.2021.112158.
- Roy R, McDonaugh B, O'Gallagher K. COVID-19 and the heart. Br Med Bull. 2022 Dec 12;144(1):4-11. doi: 10.1093/bmb/ldac022. PMID: 36155748; PMCID: PMC9619476.
- Silva NAO, Zara ALSA, Figueras A, Melo DO. Potential kidney damage associated with the use of remdesivir for COVID-19: analysis of a pharmacovigilance database. Cad Saude Publica. 2021 Nov 12;37(10):e00077721. doi: 10.1590/0102-311X00077721. PMID: 34787281.
Related Content
Neuropilin-1 joins ACE2 as SARS-CoV-2 gateway
How Nanobodies could advance Sars-CoV diagnostics
Replication Race: Scientists unravel why Omicron spreads faster
Building research careers in a post-COVID world
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.