Science paper increases understanding of how ALS and frontotemporal dementia pathology interact
Take a closer look at recent Science paper by Shao et al
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are debilitating neurodegenerative conditions that share many clinical signs, genetic hallmarks, and pathological features. One of these shared features is the cytoplasmic accumulation of TDP-43, a typically nuclear RNA-binding protein (PMID: 17023659). Despite an intense decade and a half of research since this discovery, there is still much to understand about how the biology of these two diseases is connected.
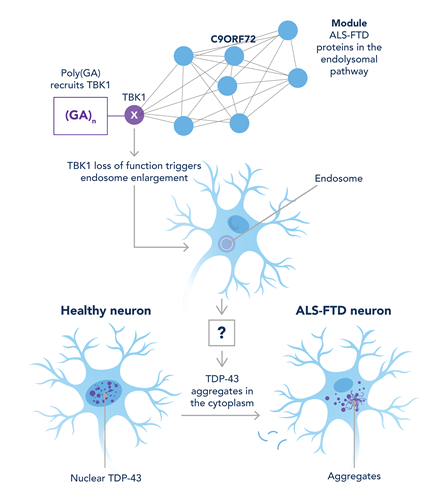
A recent paper in Science by Shao et al., “Two FTD-ALS genes converge on the endosomal pathway to induce TDP-43 pathology and degeneration,” demonstrates how C9orf72 and TBK1 interact to cause cytoplasmic accumulation of TDP-43 (PMID: 36201573). C9orf72 is a well-studied gene in relation to ALS-FTD, as when mutated, it is the most common genetic cause of these diseases. In some cases of ALS, C9orf72 obtains long hexanucleotide repeat expansions that result in the pathogenic accumulation of dipeptide repeat proteins (DPRs) in the cell via a non-canonical translation mechanism. Five DPRs are found in C9-FTD: GP repeat, GR repeat, GA repeat, AP repeat, and PR repeat. TANK-binding kinase 1 (TBK1) has been previously implicated in ALS-FTD as TBK1 loss of function mutations lead to disease (PMID: 29349657), and patients with C9orf72 and TBK1 mutations develop early onset FTD-ALS (PMID: 26674655).
Shao and colleagues revealed that phosphorylated-TBK1 alongside three of these DPRs was sequestered in the cytoplasm of mice models and human patient tissue of C9-FTD. Further investigation revealed that poly(GA) caused TBK1 to sequester in cytoplasmic intrusions causing TBK1 to become inactive and thus affect its downstream processes. Using mouse models of poly(GA) and reduced TBK1, they discovered that this relationship led to defective and enlarged endosomes and subsequent TDP43 pathology. This endosomal dysfunction supports previous studies that the endolysosomal pathway plays an important role in TDP-43 pathology, as lysosomal proteins such as progranulin (GRN) and TMEM106B are involved in disease progression.
The work of Shao and colleagues has wide implications for the field of ALS-FTD research, as it links the pathology of three important genes: TDP-43, C9orf72, and TBK1. They demonstrate how C9-poly(GA) and TBK1 lead to TDP43 pathology; this is a big step in linking genes such as TBK1 that were previously shown to be involved in FTD-ALS pathology but with no mechanistic suggestion, thus paving the way for future research in finding the pathogenetic mechanism of other implicated genes and therefore potential future therapeutic targets.
|
Figure 1: Graphic summarizing findings by Shao et al.: Poly(GA) inclusions sequester TBK1 resulting in autophosphorylation, TBK1 activity is reduced from being sequestered, disrupting endosome maturation leading to TDP-43 aggregation. Figure adapted from Gallo and Edbaur, 2022 (PMID: 36201587). |
Proteintech’s TDP-43 antibody 10782-2-AP was cited in Neuman et al.’s instrumental paper in 2006, which first discovered the role of this protein in linking the pathology of both ALS and FTD. Since then, we have developed 28 products to help researchers study TDP43 that have been cited over 1,700 times. View our TDP-43 product focus page.
Antibodies and related products for FTD-ALS research
| TDP43 related | C9orf72 related | FTD-ALS linked genes |
|
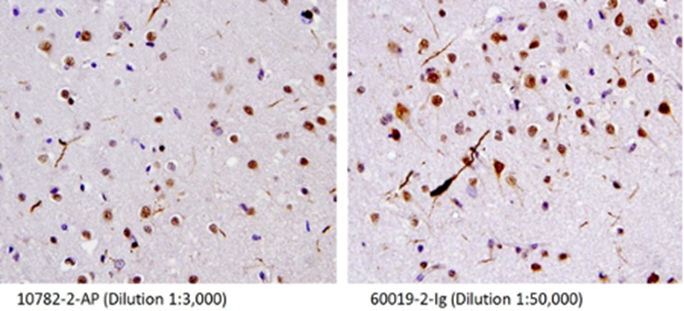
IHC staining of human brain (FTLD-U) tissue using TDP-43 PolyAB 10782-2-AP and TDP-43 (human specific) MonoAB 60019-2-Ig. 40X of FTLD-U case showing dystrophic neurites. Figures provided by Linda K. Kwong. |
Related Content
Proteintech interview with an early-career researcher: Odetta Antico
7 Tips to successfully culture primary rodent neurons

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.