Commanding Endosomal Sorting: The Role of the Commander Complex in Protein Trafficking
Written by Kincaid Ingram; PhD researcher focusing on endosomal biology in health and disease at the University of Bristol, UK
Dynamic turnover of integral membrane proteins from the cell surface is controlled by removal through endocytosis. Proteins at the plasma membrane are then repopulated from the biosynthetic pathway and endosomal recycling. Endosomal recycling machinery govern how integral proteins are sorted through the endosomal network. There are several endosomal recycling machinery, with mutations contributing to diseases such as neurodegeneration.
This article focuses on the Commander complex. It highlights research from the Collins and Cullen groups, which identified the structure of the endosomal Commander complex1. It utilizes a suite of complementary structural techniques, including X-ray crystallography, Cryo-EM, AlphaFold2 modeling, and GFP-trap immunoprecipitation, to establish and validate the Commander complex assembly.
Article based on: “The Structure of the endosomal Commander complex linked to Ritscher-Schinzel syndrome”
How the Commander Complex Regulates Protein Repopulation at the Plasma Membrane
Maintenance of the plasma membrane proteome is essential for cellular functions, including nutrient sensing and transport, cell-to-cell communication, and migration. Correct spatiotemporal control of integral membrane proteins at the cell surface is reliant on two core pathways: the biosynthetic pathway and the endolysosomal network (Figure 1). The cell integrates these pathways to regulate proteins present at the cell surface, which allows it to adapt to changing physiological demand2. Perturbations to these pathways can have detrimental effects, with links to neuronal defects3,4.
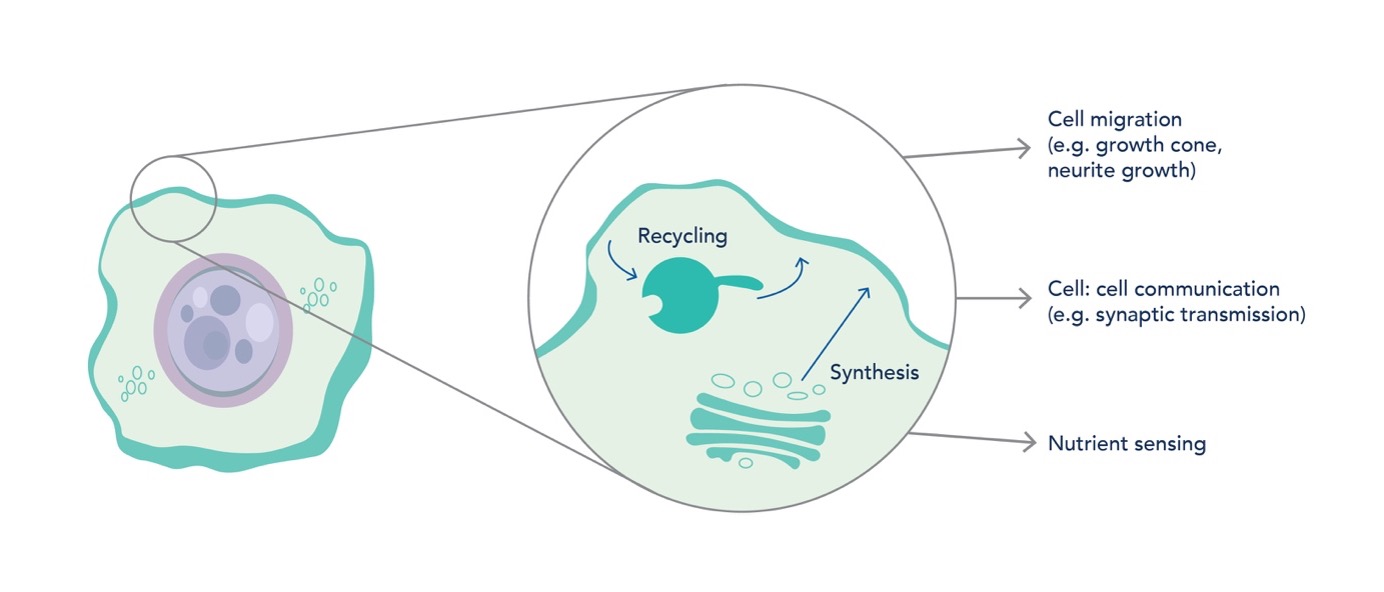
Figure 1: Protein Synthesis and Recycling Maintain the Plasma Membrane Proteome. Integral membrane proteins are removed from the cell surface via endocytosis. Re-population is mediated by the biosynthetic pathway and the endolysosomal network. Dynamic regulation of the cell surface proteome is essential for many processes, including cell migration, cell-to-cell communication, and nutrient sensing.
Integral membrane proteins and their associated lipids (termed ‘cargo’) are internalized from the plasma membrane via endocytosis, entering endocytic vesicles. Homotypic fusion of these peripheral endocytic vesicles drives formation of early endosomes, which eventually mature into late endosomes. At these endosomal compartments, cargo face a decision: lysosomal degradation or retrieval to other organelles, including the cell surface (Figure 2A). Endosomal sorting machinery orchestrate the latter, coupling sequence-dependent cargo recognition to a series of membrane remodeling events that shuttle cargo away from the endosome. Various machinery recognize and retrieve cargo, enriching them at the endosomal retrieval subdomain in preparation for recycling, for instance the Retromer and Commander complexes5–7 (Figure 2B).
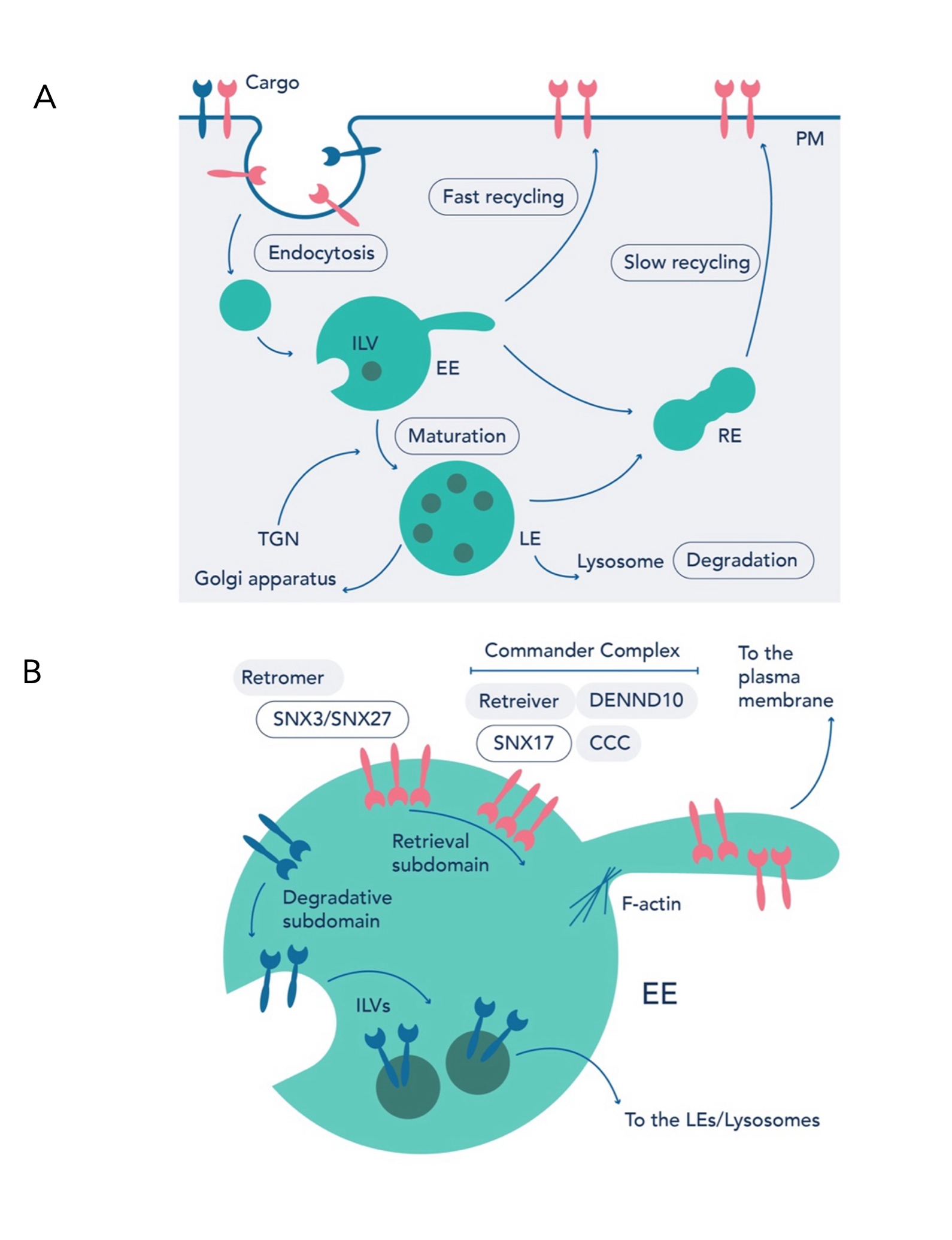
Figure 2. The endolysosomal network. (A) Transmembrane proteins (termed ‘cargo’) are delivered to the cell surface via the secretory pathway. Nascently internalized cargo from the cell surface enter endocytic vesicles. These endocytic vesicles fuse to form EEs. EEs mature through the endolysosomal network to LEs and finally lysosomes for degradation. Cargo destined for degradation are packaged into ILVs. Alternatively, cargo can be returned to the PM or TGN, preventing degradation in the lysosome. (B) Endosomal sorting machinery recruit cargo to the retrieval subdomain. Cargo adaptors, such as (SNX3)/ SNX27 or SNX17, work in tandem with Retromer or Commander, respectively, to enrich cargo. These machinery couple sequence dependent cargo recognition and membrane remodeling to package cargo into tubulovesicular carriers for endosome exit. CCC, COMMD/CCDC22/CCDC93; DENND10, Differentially expressed in normal and neoplastic cells-containing protein 10; EEs, early endosomes; ILVs, intra-luminal vesicles; LEs, late endosomes; PM, plasma membrane; SNX, sorting nexin; TGN, trans-Golgi network.
Breaking Down the Commander Complex: Insights into its Subunit Structure
The Commander complex contains 16 subunits, constructed in two sub-assemblies: the Retriever and CCC complexes. Retriever is a heterotrimer comprised of vacuolar protein sorting 26C (VPS26C):VPS35L:VPS29, that shares distant homology with another endosomal recycling complex Retromer8. Retromer is a heterotrimer of VPS26A or VPS26B:VPS35:VPS29. The CCC complex contains twelve subunits, ten COMMD (copper metabolism MURR1 [Mouse U2af1-rs1 region 1] domain) family members COMMD1-COMMD10, plus two coiled-coil domain-containing proteins CCDC22 and CCDC93. DENND10 (Differentially expressed in normal and neoplastic cells-containing protein 10), also called FAM45A, is the final subunit (Figure 3A). Mutations within the complex are causative for X-linked intellectual disability (XLID)9 and Ritscher-Schinzel syndrome (RSS), a multisystem developmental disorder10–14.
Commander regulates recycling of hundreds of integral proteins. The majority of cargo sorted by Commander, including ɑ5ß1 integrin and amyloid precursor protein (APP), contain a ϕxNxx[YF] motif (ϕ is any hydrophobic amino acid) that are recruited through a cargo adaptor, sorting nexin 17 (SNX17)8,15,16.
Healy, McNally, Butkovič, Chilton, and colleagues present a complete model of the Commander complex 1. The authors modeled the complete complex with AlphaFold2 and resolved areas with X-ray crystallography and cryo-electron microscopy (cryo-EM). This smorgasbord of structural techniques allowed the authors to delineate the 3D assembly of the 16 subunits. They present the following data (Figure 3B):
- Retriever assembles into a complex with unique features that allow for its divergent function from Retromer.
- The ten COMMD proteins assemble into a heterodecameric ring structure comprised of five obligate dimers.
- CCDC22 and CCDC93 stabilize the CCC complex, with the N-termini forming extensive interactions with the decameric COMMD The two CCDC proteins link Retriever to the COMMD ring, while recruiting DENND10 via a central coiled-coil structure.
These data provide further understanding of the Commander complex and hence insight into the assembly and function required for recycling of essential cargo.

Figure 3. The Commander complex assembly. Constituents (A) and 3D structure (B) of the Commander complex. The Commander complex is a 16-subunit assembly. It comprises of two sub-assemblies: Retriever and CCC complex. Retriever is a heterotrimer of VPS26C:VPS35L:VPS29. The CCC complex contains twelve subunits: CCDC22, CCDC93 and ten COMMDs. DENND10 is the 16th subunit (article Figure 1A & 6A, respectively). CCC, COMMD/CCDC22/CCDC93; CCDC, coiled-coil domain-containing protein; COMMD, copper metabolism MURR1 [Mouse U2af1-rs1 region 1] domain; DENND10, Differentially expressed in normal and neoplastic cells-containing protein 10; VPS, vacuolar protein sorting.
The Unique Structure and Function of Retriever
Initially, the authors validated Retriever’s 1:1:1 stoichiometry (VPS26C:VPS35L:VPS29). Human Retriever was recombinantly expressed in insect cells and isolated via affinity purification and size-exclusion chromatography. Subsequent negative stain EM and 2D/3D cryo-EM classes distinguished a ‘footprint’ orientation of the heterotrimer. Due to the propensity for particles to be in this ‘footprint’ orientation, there was limited data to begin 3D reconstructions. However, a high-confidence AlphaFold2 model aligned well with the low-resolution 3D cryo-EM data (Figure 4A).
To validate the accuracy of the AlphaFold2 model, Healy and coauthors utilized site-directed mutagenesis of key residues at the proposed binding interfaces. The authors transiently overexpressed GFP-tagged chimeric proteins (GFP-protein) in HEK293T cells. After cell lysis, GFP-nanotrap immunoprecipitation (IP) was used to enrich the GFP-protein on agarose beads. Protein samples were then resolved on Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gels and probed via western blot. IP identified and compared interacting proteins pulled down with wild-type and mutant proteins (Figure 4B). A whole suite of VPS35L and VPS29 mutants were used to scrutinize the Retriever model and validate the accuracy of predicted binding interfaces (Figure 4B).
VPS35L has an extended ɑ-solenoid structure that binds VPS26C and VPS29 at its N- and C-termini, respectively. VPS26C:VPS35L interaction is mediated by VPS35L 2nd and 3rd ɑ-helical repeats binding to the VPS26C C-terminal ß-sandwich domain. The authors generate VPS35L point mutants to tease apart the direct interactions at this interface. IP of VPS35L-GFP WT shows robust interaction with Retriever (VPS26C and VPS29) and CCC components (CCDC22 and CCDC93). However, A285D and R293E point mutants have a partial and complete reduction of VPS26C binding, respectively (Figure 4B). Importantly, binding to CCC components remains unperturbed with these mutants.
In comparison to Retromer, Retriever has two unique interactions that promote VPS29 binding and regulate its function. First, VPS35L has a ß-sheet extension that contacts VPS29. Perturbation of this binding region reduced VPS29 interaction; however, it retained CCC complex binding. Second, VPS35L contains an extended N-terminal region that contacts VPS29. This region wraps around VPS29 like a seat belt, aptly named the ‘belt’ region17, and stabilizes the complex (Figure 4A). Mutations to this region result in loss of both VPS29 and CCC complex interactions.
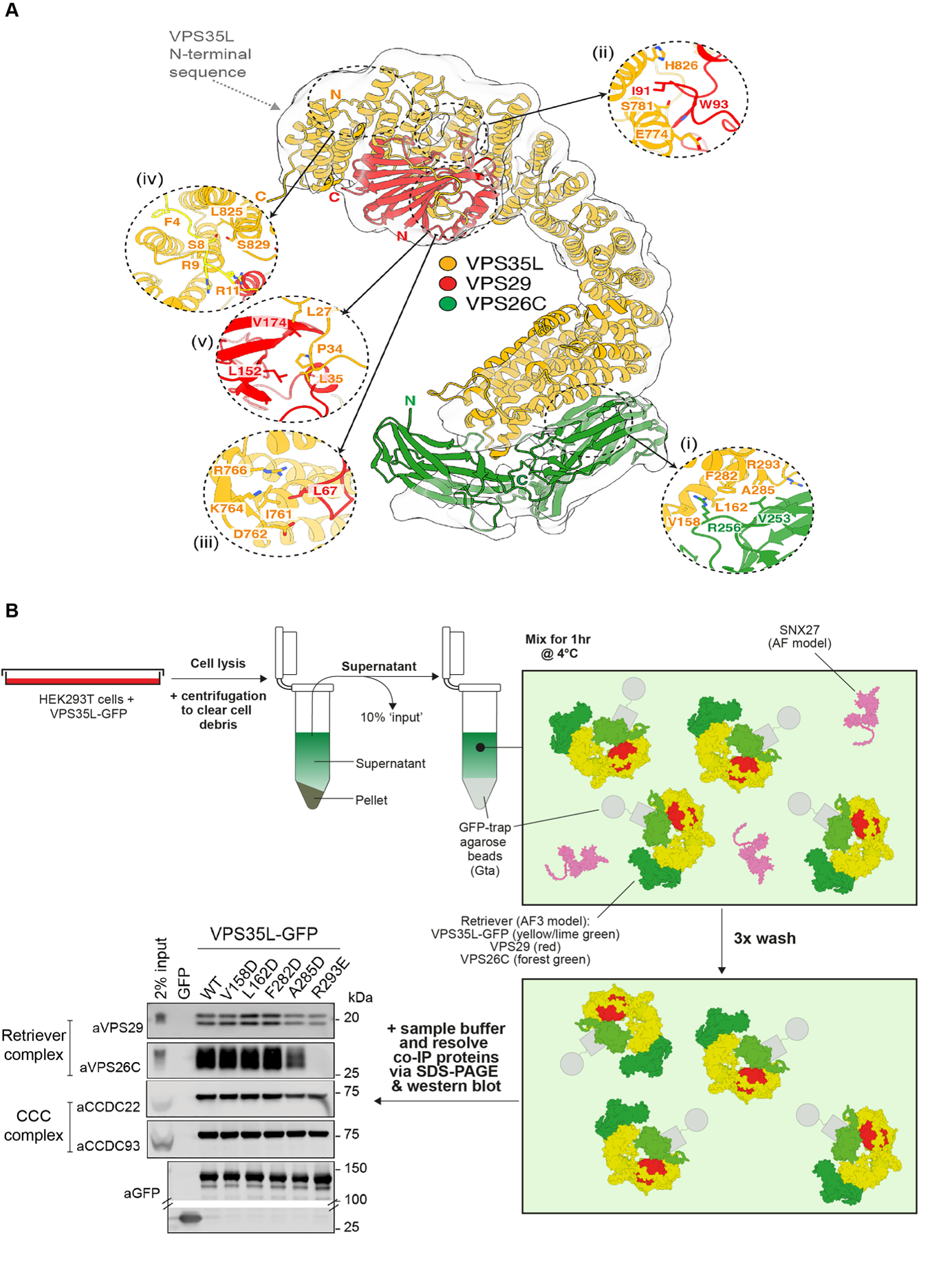
Figure 4. Retriever AlphaFold2 model is validated by structure-based mutagenesis. (A) Retriever is a heterotrimer of VPS26C (green):VPS35L (yellow):VPS29 (red). High confidence AlphaFold2 model superimposed on low-resolution 3D cryo-EM envelope. Key heterotrimer binding interfaces are highlighted (i-v) (article figure 1B). (B) GFP-nanotrap immunoprecipitation allowed authors to pick apart Retriever binding interface. HEK293T cells transiently transfected with VPS35L-GFP. Cells lysed and VPS35L-GFP enriched via immunoprecipitation. SNX27 model taken from AlphaFold database (UniProt Accession: Q96L92). Retriever model , including VPS35L-GFP, modeled using AF3. Interactors are probed for via western blotting. VPS35L and VPS26C binding interface validated via GFP-nanotrap immunoprecipitation (western blot taken from article). Proteintech aCCDC22 (16636-1-AP) and aCCDC93 (20861-1-AP) used to test VPS35L mutant impact on Retriever-CCC complex binding (adapted from article figure 1C). AF, AlphaFold; AF3, AlphaFold3; SNX27, sorting nexin-27.
Understanding the COMMD Ring Assembly
Cryo-EM structural analysis revealed the human COMMD proteins assemble into a distinct hetero-decameric closed ring. The ring is comprised of five heterodimers connected in precise order ((COMMD1-6)-(COMMD4-8)-(COMMD2-3)-(COMMD10-5)-(COMMD7-9)). The COMMD proteins assemble into something analogous to a ‘bagel’ (unofficial term). The COMM domains resemble the filling of the bagel. The a-helical N-terminal (HN) domains decorate the upper and lower surfaces of the structure, like the slices of bread (Figure 5).
CCDC22 and CCDC93 make extensive contacts with the COMMD ring. Cryo-EM analysis revealed linker regions of the CCDC proteins intertwined within the COMMD ring structure (Figure 5). These data suggest that the complex will strictly function as a dodecamer within a cellular environment.
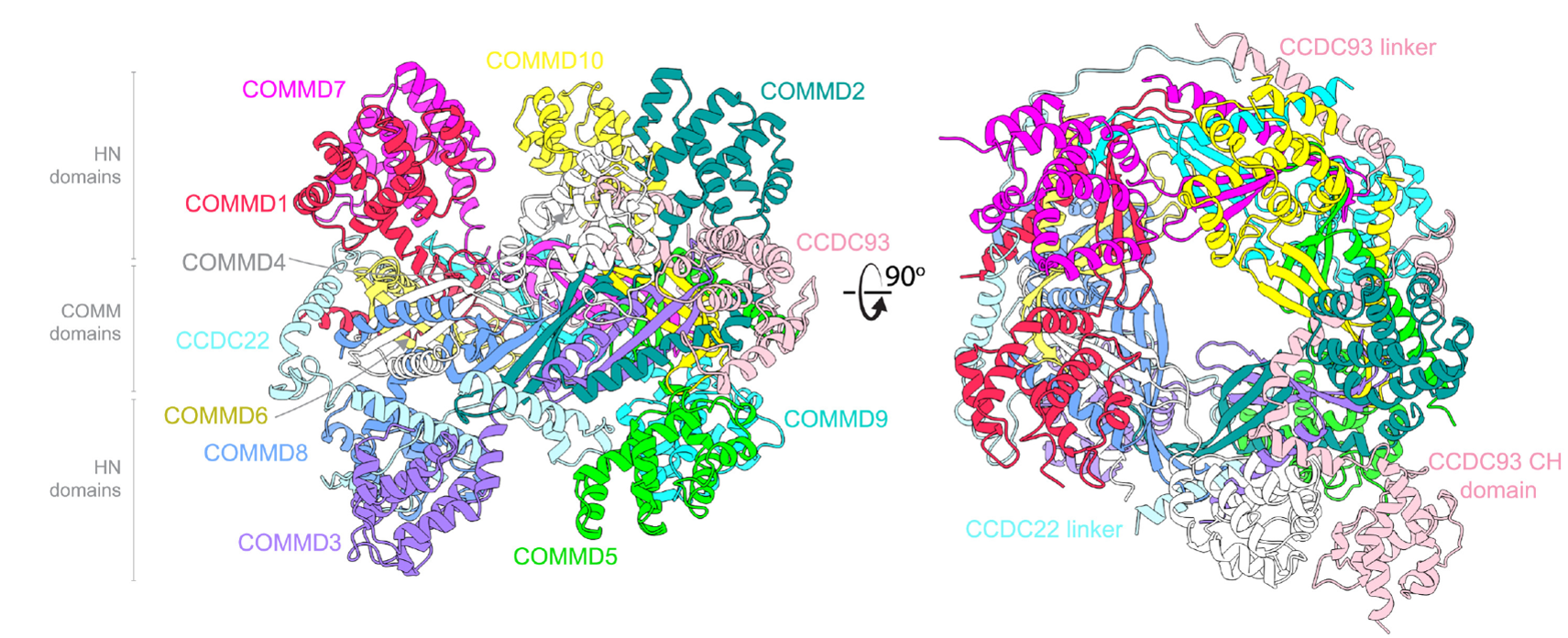
Figure 5. CCC complex Cryo-EM structure. COMMD ring assembled of obligate dimers. Central to the COMMD ring are the COMM domains, with the HN domain decorating the upper and lower surfaces of the structure. CCDC93 and CCDC22 linker domains wrap around the structure. CCDC93 CH domain also visible (article figure 4A). CCDC, coiled-coil domain-containing protein; CH, calponin-homology; COMMD, copper metabolism MURR1 [Mouse U2af1-rs1 region 1] domain; HN, a-helical N-terminal.
Deciphering the Commander Holo-Complex Using AlphaFold2 and Structural Biology
The authors combine AlphaFold2 models and experimental data to piece together a structure for the 16-subunit Commander complex. The CCDC proteins form a heterodimer with four coiled-coil regions. These coiled-coils assemble into two V-shaped structures. Nested at the apex of one ‘V’ is DENND10. At other regions of the CCDC22-CCDC93 heterodimer Retriever and the COMMD ring bind. The heterodimer interacts with Retriever through a conserved region in VPS35L, opposite the site occupied by VPS29. CCDC22-CCDC93 bind to the COMMD ring extensively, as discussed, tethering the Retriever and COMMD complexes together (Figure 3B).
Finally, the authors explore the consequences of XLID and RSS causative mutations. The majority of missense mutations map to inter-subunit interfaces or key structural elements. The mutations tested all resulted in a reduction of Commander protein levels. Additionally, there were frameshift and deletion mutations in VPS35L. These mutations impacted VPS29 binding as they were proximal to the interaction interface. Importantly, these VPS35L mutations did not dramatically impact overall complex formation.
Conclusion
Spatiotemporal control of cargo flux through the endolysosomal network is critical to cellular function. Endosomal sorting machinery are vital for cargo exit from the network. The Commander complex coordinates the retrieval of numerous integral proteins. Commander is a 16-protein complex comprised of two sub-assemblies: Retriever and the CCC complex. Commander function is essential to cellular homeostasis, with mutations linked to various developmental disorders.
Healy, McNally, Butkovič, Chilton, and colleagues provide a comprehensive understanding of the Commander holo-complex1. The authors utilize in silico, in vitro, and in cellulo techniques to dissect its structure. These data provide a molecular explanation for the coupling of the CCC complex and Retriever into the Commander assembly. Also, it illuminates the molecular basis for XLID and RSS disease-causing mutations.
These findings have been further validated by later publications17,18 and all three have been reviewed together19.
Products
Proteintech products used in this study.
|
Product |
Cat No. |
|
GFP-trap |
|
|
mCherry-trap |
|
|
aCCDC22 |
|
|
aCCDC93 |
|
|
aCOMMD1 |
|
|
aCOMMD5 |
|
|
aCOMMD8 |
References
- Healy, M. D. et al. Structure of the endosomal Commander complex linked to Ritscher-Schinzel syndrome. Cell 186, 2219-2237.e29 (2023).
- Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol 19, 679–696 (2018).
- Small, S. A., Simoes-Spassov, S., Mayeux, R. & Petsko, G. A. Endosomal Traffic Jams Represent a Pathogenic Hub and Therapeutic Target in Alzheimer’s Disease. Trends Neurosci 40, 592–602 (2017).
- Herman, M., Randall, G. W., Spiegel, J. L., Maldonado, D. J. & Simoes, S. Endo-lysosomal dysfunction in neurodegenerative diseases: opinion on current progress and future direction in the use of exosomes as biomarkers. Philosophical Transactions of the Royal Society B: Biological Sciences 379, (2024).
- Mallam, A. L. & Marcotte, E. M. Systems-wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development. Cell Syst 4, 483–494 (2017).
- McNally, K. E. & Cullen, P. J. Endosomal Retrieval of Cargo: Retromer Is Not Alone. Trends Cell Biol 28, 807–822 (2018).
- Weeratunga, S., Paul, B. & Collins, B. M. Recognising the signals for endosomal trafficking. Current Opinion in Cell Biology vol. 65 Preprint at https://doi.org/10.1016/j.ceb.2020.02.005 (2020).
- McNally, K. E. et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol 19, 1214–1225 (2017).
- Voineagu, I. et al. CCDC22: a novel candidate gene for syndromic X-linked intellectual disability. Mol Psychiatry 17, 4–7 (2012).
- Kato, K. et al. The congenital multiple organ malformation syndrome, Ritscher-Schinzel syndrome is an endosomal recyclinopathy. Preprint at https://doi.org/10.1101/2024.08.17.24311658 (2024).
- Kato, K. et al. Biallelic VPS35L pathogenic variants cause 3C/Ritscher-Schinzel-like syndrome through dysfunction of retriever complex. J Med Genet 57, 245–253 (2020).
- Otsuji, S. et al. Clinical diversity and molecular mechanism of VPS35L-associated Ritscher-Schinzel syndrome. J Med Genet 60, 359–367 (2023).
- Elliott, A. M. et al. A novel mutation in KIAA0196 : identification of a gene involved in Ritscher–Schinzel/3C syndrome in a First Nations cohort. J Med Genet 50, 819–822 (2013).
- Kolanczyk, M. et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher-Schinzel/3C syndrome. European Journal of Human Genetics 23, 633–638 (2015).
- Ghai, R. et al. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proceedings of the National Academy of Sciences 110, (2013).
- Butkovič, R. et al. Mechanism and regulation of cargo entry into the Commander endosomal recycling pathway. Nat Commun 15, (2024).
- Boesch, D. J. et al. Structural organization of the retriever–CCC endosomal recycling complex. Nat Struct Mol Biol (2023) doi:10.1038/s41594-023-01184-4.
- Laulumaa, S., Kumpula, E.-P., Huiskonen, J. T. & Varjosalo, M. Structure and interactions of the endogenous human Commander complex. Nat Struct Mol Biol (2024) doi:10.1038/s41594-024-01246-1.
- Leneva, N. & Kovtun, O. The commander complex is the Swiss Army knife of endosomal trafficking. Nat Struct Mol Biol (2024) doi:10.1038/s41594-024-01326-2.
Related Content
Blog: Exosomes: A big role for a small player
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

