Markers for Astrocyte Identification
Written by Haddie DeHart, PhD student at Indiana University School of Medicine
Introduction:
Astrocytes are star-shaped glial cells in the central nervous system (CNS) that are critical to brain and spinal cord function. Previously thought to function solely as support for neurons, astrocytes also play essential roles in regulating the blood-brain barrier, balancing ions, and clearing neurotransmitters. They also account for approximately 50% of all cells in the brain. Their involvement in normal brain function and neurological diseases has brought them to the forefront of research, highlighting the need for astrocyte-specific markers that will allow for the identification and further scrutiny of the biological roles of these glial cells.
Common astrocyte markers:
To label astrocytes, antibodies against specific astrocytic proteins are commonly used in immunoassays such as western blot, immunohistochemistry, and immunofluorescence. Popular astrocyte markers include:
GFAP (Glial Fibrillary Acidic Protein)
GFAP is a well-known intermediate filament protein that plays a key role in cytoskeletal support. It is primarily expressed in astrocytes, making it the gold standard marker for mature astrocytes. Additionally, GFAP expression is particularly useful for identifying reactive astrocytes, as its levels increase in response to CNS injuries, inflammation, and neurodegenerative diseases. However, it is important to note that GFAP can also be upregulated in other cell types in certain pathological conditions. Therefore, using multiple markers or conducting morphological analyses might be required to distinguish astrocytes from other cells.
Proteintech offers conjugated and unconjugated GFAP antibodies suitable for various applications.
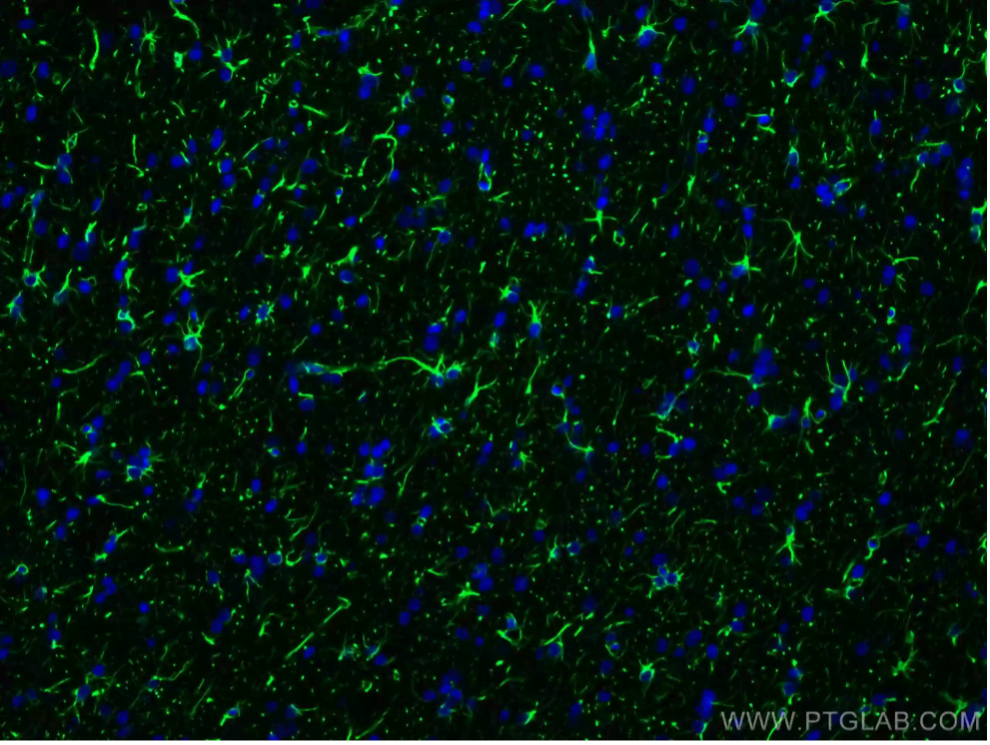
Figure 1: Immunofluorescent analysis of 4% PFA-fixed rat brain tissue using GFAP antibody (81063-1-RR) at a dilution of 1:200 and CoraLite 488-conjugated Goat Anti-Rabbit IgG (H+L).
S100β (S100 Calcium-Binding Protein)
S100β is a calcium-binding protein that plays an essential role in calcium signaling, neuroprotection, and injury response. The protein is expressed predominantly in astrocytes, at both immature and mature stages, making it an informative marker for studies in healthy and diseased brain tissues. Levels of S100β can also be analyzed to identify the reactiveness of astrocytes in certain conditions.
Proteintech offers antibodies and kits for IHC and ELISA against S100β.
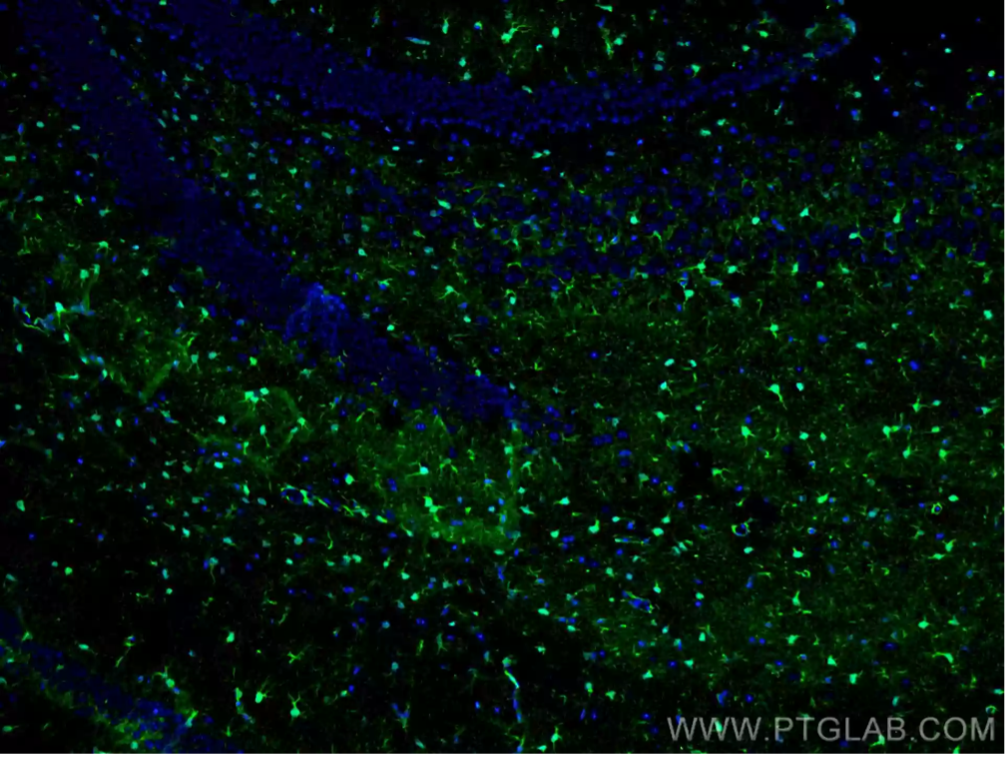
Figure 2: Immunofluorescent analysis of 4% PFA-fixed mouse brain tissue using S100 Beta antibody (15146-1-AP) at a dilution of 1:200 and CoraLite 488-conjugated Goat Anti-Rabbit IgG (H+L).
ALDH1L1 (Aldehyde Dehydrogenase 1 Family Member L1)
Genetic profiling of astrocytes has led to the identification of a broader range of astrocyte markers, such as ALDH1L1. This protein plays key roles in cellular metabolism, particularly in detoxifying aldehydes and participating in folate metabolism. Known as a pan-astrocyte marker in the mature brain, ALDH1L1 is highly specific to astrocytes, making it ideal for conditions with mixed glial populations.
Proteintech offers stringently validated monoclonal and polyclonal antibodies for ALDH1L1.
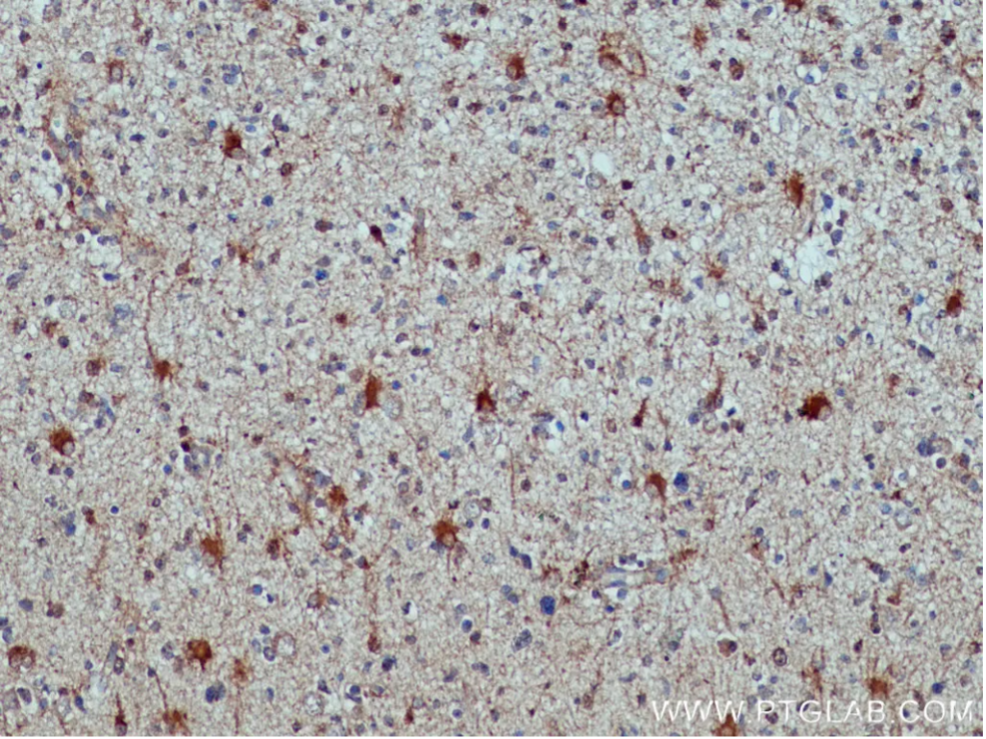
Figure 3: Immunohistochemical analysis of paraffin-embedded human glioma tissue slide using ALDH1L1 antibody (17390-1-AP) at a dilution of 1:200. Heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) was used.
SOX9 (SRY-Box Containing Gene 9)
During neural development, SOX9 drives the differentiation of neural progenitor cells into astrocytes, marking its pivotal role in establishing and maintaining the astrocyte lineage. In mature astrocytes, SOX9 regulates genes linked to glial identity and supports cellular functions that promote homeostasis and neural support. Its specific expression in astrocytes, compared to neurons or other glial cells, makes SOX9 a reliable marker for studies investigating astrocyte populations, differentiation, and reactivity in both development and disease contexts.
Validated in western blot, IHC, and flow cytometry, Proteintech offers highly sensitive antibodies for SOX9.
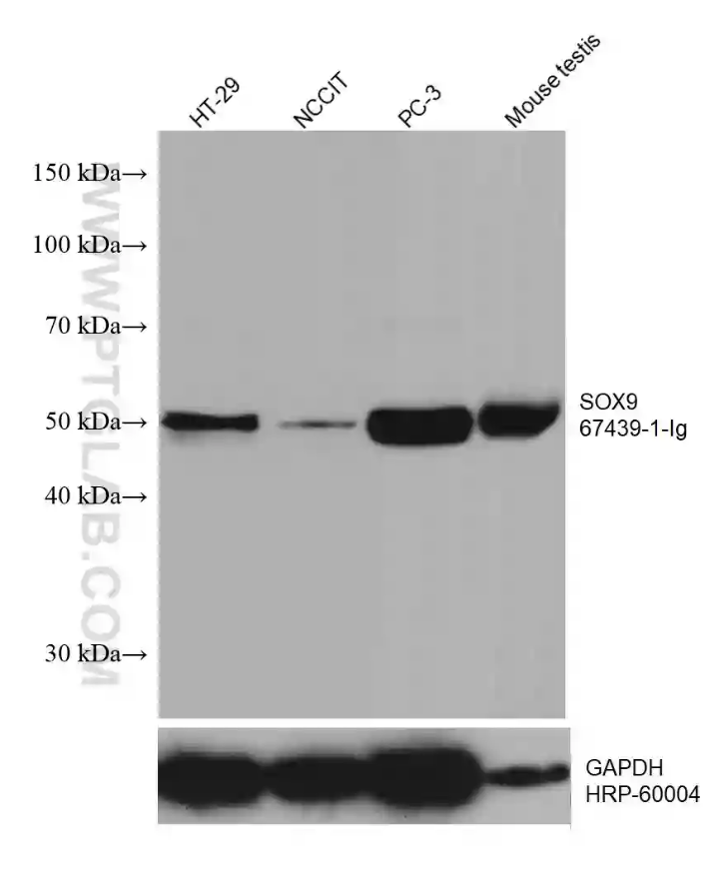
Figure 4: Various lysates were subjected to SDS PAGE followed by western blot with the SOX9 antibody (67439-1-Ig) at a dilution of 1:10,000, incubated at room temperature for 1.5 hours. The membrane was stripped and reblotted with HRP-conjugated GAPDH monoclonal antibody as a loading control.
Other Markers
Depending on research context, other markers may be used to study astrocyte function and morphology.
-
-
EAAT2 (Excitatory Amino Acid Transporter 2, also known as GL-T1)
-
AQP4 (Aquaporin 4)
-
CX43 (Connexin 43)
-
NF-κB (Nuclear Factor Kappa-light-chain-enhancer of Activated B cells)
-
IL-6 (Interleukin 6)
-
IL-1β (Interleukin 1 Beta)
-
TNF-α (Tumor Necrosis Factor Alpha)
-
CCL2 (C-C Motif Chemokine Ligand 2, also known as MCP-1)
-
CXCL10 (C-X-C Motif Chemokine Ligand 10, also known as IP-10)
-
CXCL12 (C-X-C Motif Chemokine Ligand 12, also known as SDF-1)
-
|
Pathology |
Astrocyte Response |
|
Alzheimer’s Disease (AD) & Parkinson’s Disease (PD) |
· Amyloid-β and tau pathology suppress EAAT2 expression, leading to glutamate dysregulation and synaptic toxicity. · Loss of perivascular AQP4 polarization impairs cerebrospinal fluid (CSF) flow, leading to AD. · Aβ deposits induce CX43 upregulation, disrupting astrocytic potassium and glutamate buffering, leading to synaptic hyperactivity and neurodegeneration. · NF-κB is hyperactivated in reactive astrocytes in AD and PD, leading to the production of pro-inflammatory cytokines (IL-1β, TNF-α), exacerbating neuronal damage. |
|
Amyotrophic Lateral Sclerosis (ALS) |
· EAAT2 is selectively downregulated, leading to glutamate accumulation and motor neuron death. |
|
Brain Cancer · Glioblastoma (GBM) · High-Grade Glioma · Astrocytoma · Medulloblastoma (MB) |
· Increased AQP4 expression promotes cellular water flux, allowing tumor cells to rapidly adapt to microenvironmental changes and invade surrounding tissues. · CX43 is highly expressed in brain cancer, where it facilitates tumor growth and invasion. · CCL2 & CXCL12 recruit tumor-associated macrophages (TAMs) and microglia, promoting an immunosuppressive TME. Whereas IL-6 & TNF-α drive glioma stem cell maintenance and radioresistance. · Vimentin, along with GFAP, is upregulated in tumor associated astrocytes and used as a marker for reactive astrocytes. · Tumor cells activate NF-κB in astrocytes, leading to the release of pro-tumorigenic cytokines like IL-6, IL-1β, and TNF-α. · NF-κB activation in astrocytes promotes cancer cell proliferation and therapy resistance. |
|
Ischemic stroke |
· EAAT1/EAAT2 dysfunction leads to glutamate accumulation, causing reduced transporter function, resulting in neuronal loss. · AQP4 is upregulated, causing astrocytes to swell and increasing intracranial pressure, leading to neuronal damage. · Hypoxia increases CX43 phosphorylation, promoting hemichannel opening, cell swelling, and inflammation. |
|
Multiple Sclerosis (MS) |
· CX43 blockade reduces neuroinflammation and improves remyelination in MS models. · NF-κB upregulates CXCL10 and CCL2, promoting T cell infiltration into the CNS. |
|
Neuromyelitis Optica Spectrum Disorder (NMOSD) |
· NMO-IgG (anti-AQP4 antibody) binding to AQP4 triggers astrocyte destruction, inflammatory cell infiltration, and demyelination. |
|
Schizophrenia and Mood Disorders |
· EAAT1/EAAT2 dysfunction is associated with altered glutamate homeostasis. |
|
Strokes, Seizures, Epilepsy & Neurotrauma |
· Dysfunction of EAAT1/EAAT2 leads to prolonged excitatory neurotransmission, increasing seizure susceptibility. · AQP4 interacts with EAAT2, an astrocytic glutamate transporter. AQP4 dysfunction reduces glutamate uptake, promoting hyperexcitability. · CX43-deficient mice exhibit reduced seizure susceptibility. · Vimentin is upregulated in reactive astrocytes following brain injury. It facilitates astrocyte hypertrophy and glial scar formation. · Excessive IL-1β and TNF-α secretion exacerbates excitotoxicity by disrupting astrocytic glutamate uptake. · Secreted cytokines like IL-6, TNF-α, and CXCL10 weaken tight junctions, increasing BBB permeability. |
Key Aspects to Consider When Selecting Markers
As with any investigation, it is important to understand the context of your work and choose the marker that best suits your study. Here are a few considerations:
- Where do the astrocytes originate?
- Molecular and functional studies have shown astrocytes are heterogeneous across the brain.
- For example, in mice, GFAP is more highly expressed in the hippocampus and midbrain, while S100β is more prominently expressed in the cerebrum and hindbrain.
- What biological aspects of astrocytes am I studying?
- If the goal is to study astrocyte development, one might focus on S100β and EAAT1 expression, as GFAP is typically more abundant in mature/differentiated astrocytes.
- What species will I work with?
- Consider species-specific differences, such as those between human, mouse, or rat astrocytes.
- In a paper published in 2021 by Li et al., they compared the transcriptome of human and mouse astrocytes. While a large proportion of gene expression was conserved, mice showed higher expression for genes related to metabolism and the mitochondria, while human astrocytes expressed genes related to defense response and proteins destined for the extracellular space.
- Is the chosen marker expressed by other cells in the environment?
- While S100β is a common astrocyte marker, it also identifies oligodendrocytes and oligodendrocyte progenitor cells. Similarly, EAAT2 is detectable in some neurons.
- When studying astrocytes in the context of glioblastoma (GBM), GAPDH can still be used as its expression has been shown to decrease as the grade of the astrocytoma increases.
- What assays will I use to identify my astrocytes? What technical aspects should I consider?
- While immunofluorescence (IF) is one of the most widely used techniques for studying astrocyte morphology, other applications, based on experimental approach or resource availability, may also be used.
- For example, a GFAP antibody validated for western blot analysis, IF, and immunohistochemistry (IHC), would allow researchers to look at GFAP protein expression, examine the morphology of an astrocyte in culture or in organoids, and determine the number of astrocytes present in patient tumor samples, all with one antibody.
Citations
- Yang Z, Wang KKW. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends in Neuroscience. 2015;38(6):364-374. doi:10.1016/j.tins.2015.04.003
- Schiweck J, Eickholt BJ, Murk K. Important shapeshifter: Mechanisms Allowing Astrocytes to Respond to the Changing Nervous System During Development, Injury and Disease. Frontiers in Cellular Neuroscience. 2018;12. doi:10.3389/fncel.2018.00261
- Zhang H, Zhou Y, Cui B, Liu Z, Shen H. Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomedicine & Pharmacotherapy. 2020;126. doi:10.1016/j.biopha.2020.110086
- Endo F, Kasai A, Soto JS, et al. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science. 2022;378(6619). doi:10.1126/science.adc9020
- Guttenplan KA, Liddelow SA. Astrocytes and microglia: Models and tools. The Journal of Experimental Medicine. 2019;216(1):71-83. doi:10.1084/jem.20180200
- Brandao M, Simon T, Critchley G, Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia. 2019;67(5):779-790. doi:10.1002/glia.23520
- Mega A, Hartmark Nilsen M, Leiss LW, et al. Astrocytes enhance glioblastoma growth. Glia. 2020;68(2):316-327. doi:10.1002/glia.23718
- Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. doi:10.1038/nature21029
- Yu X, Nagai J, Khakh BS. Improved tools to study astrocytes. Nature Reviews Neuroscience. 2020;21(3):121-138. doi:10.1038/s41583-020-0264-8
- Zhang Y, Chen K, Sloan SA, et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. Journal of Neuroscience. 2014;34(36):11929-11947. doi:10.1523/JNEUROSCI.1860-14.2014
- Oberheim NA, Takano T, Han X, et al. Uniquely Hominid Features of Adult Human Astrocytes. Journal of Neuroscience. 2009;29(10):3276-3287. doi:10.1523/JNEUROSCI.4707-08.2009
- Li J, Pan L, Pembroke WG, et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nature Communications. 2021;12(1):3958. doi:10.1038/s41467-021-24232-3
Related Content
Identifying Cortical Cell Types Through Immunofluorescence and Electrophysiology
CoraLite conjugated antibodies
A Guide to Immunostaining the Cerebellum
How to choose the right secondary antibody
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.