Halotag
HaloTag is a protein tag. The HaloTag protein can be fused to your protein of interest (POI) which enables it for cellular and biochemical analysis. It can also be used for live cell imaging.
- What is HaloTag?
- How does HaloTag work?
- How is the complex between the HaloTag and a ligand formed?
- What size is the HaloTag?
- For what applications can HaloTag be used?
- Which ligands are available?
- Is there more than one HaloTag version available?
- What are the differences between HaloTag and Snap-Tag?
- What are the differences between HaloTag and GFP?
- How can I detect my Halo-tagged protein in Western blot?
- How can I immunoprecipitate my Halo-tagged protein?
- Can I pulldown a HaloTag fusion protein, that is already bound to a substrate?
- How can I elute my HaloTag fusion protein?
- What are the binding kinetics of the Halo-Trap?
- What are excitation & emission wavelengths of HaloTag?
What is HaloTag?
The HaloTag is a self-labeling protein derived from the haloalkane dehalogenase enzyme DhaA from Rhodococcus rhodochrous. It can be used as protein tag for tagging your protein of interest (POI).
How does HaloTag work?
After transfection of your cell line/organism with a plasmid encoding for your Halo-tagged protein, the Halo-tagged protein is expressed. Through incubation of your cell line/organism with specific ligands (see below point 6), your Halo fusion protein
-
- turns into a fluorescent protein by binding a fluorescent dye or
- acquires biotin for immobilization or other streptavidin modifications.
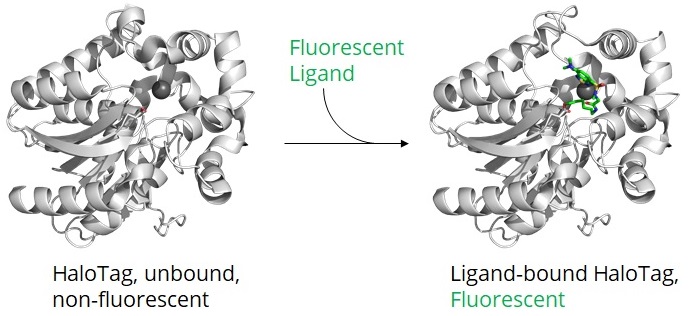
Once the HaloTag has formed a complex with a ligand, the catalytic center is not accessible for additional ligands. Alternatively, the Halo fusion protein can be detected or immunoprecipitated by antibodies or Nanobodies binding outside of its catalytic center (see below point 10, 11, 12).
How is the complex between the HaloTag and a ligand formed?
As mentioned above, the HaloTag is derived from haloalkane dehalogenase enzyme DhaA. Its active site has been genetically modified. Chloroalkane linker substrates are irreversibly bound to the active site. Biotin, Alexa Fluor® 488 or TMR with chloroalkane linker are commercially available.
What size is the HaloTag?
The HaloTag consists of 297 amino acids and has a molecular weight of 33 kDa.
For what applications can HaloTag be used?
The HaloTag is typically used for
-
- cell-based/imaging assays such as immunofluorescence
- immunoprecipitation
- protein purification.
Which ligands are available?
There are fluorescent dyes as ligands for microscopy available, which are cell permeable (TMR, Coumarin) or cell impermeable such as Alexa Fluor 488. In addition, there are Biotin and PEG-Biotin available.
Is there more than one HaloTag version available?
The HaloTag version that is currently commercially available is HaloTag7. However, a version HaloTag2 was previously sold. HaloTag2 and HaloTag7 differ in about 17 amino acids, which are partly positioned on the proteins surface.
What are the differences between HaloTag and Snap-Tag?
Snap-Tag is also an extrinsically fluorescent protein tag that require the capture of fluorescent ligands to turn into a fluorescent protein. In contrast to HaloTag, SNAP-tag is derived from the human O6-alkylguanine-DNA-alkyltransferase (hATG). SNAP-tag reacts covalently with O6-benzylguanine derivatives in an irreversible and highly specific manner.
Erdmann et al. (2019), compared HaloTag and Snap-Tag in live cell imaging with silicone rhodamine derivatives (=SiR-based dyes). They conclude that “for single-color imaging of SiR-based dyes , by either confocal or STED microscopy, we recommend Halo tagging as a first choice, since it provides fluorescence that is brighter and less prone to bleaching rapidly.”
|
Property |
HaloTag |
SNAP-Tag |
|
Discovery/ first publication |
2008 |
2008 |
|
Origin |
Haloalkane dehalogenase from Rhodococcus rhodochrous |
human O6-alkylguanine-DNA-alkyltransferase |
|
Structure |
Monomer |
Monomer |
|
Reactivity |
Chloroalkane derivatives |
O6-benzylguanine derivatives |
|
Ligands |
Cell permeable or non-permeable dyes, fluorophores, biotin, and beads |
Cell permeable or non-permeable dyes, fluorophores, biotin, and beads |
|
Excitation/ Emission maximum |
Depends on coupled fluorophore |
Depends on coupled fluorophore |
|
Length/ molecular weight (MW) |
297 amino acids, |
182 amino acids, |
What are the differences between HaloTag and GFP?
GFP is an intrinsically fluorescent protein. The fluorescence arises through the protein itself, when excited with light (Technically speaking, GFP forms a β-barrel structure. The fluorescence comes from the backbone cyclization and oxidation of three amino acid residues in the middle of the barrel and results in a two-ring chromophore). There is no need for adding substrates or ligands. Photostability and quantum yield is normally lower compared to chemical dyes.
How can I detect my Halo-tagged protein in Western blot?
ChromoTek offers a validated, sensitive anti-Halo mouse monoclonal antibody for Western blotting.
How can I immunoprecipitate my Halo-tagged protein?
ChromoTek offers Halo-Trap Agarose and Halo-Trap Magnetic Agarose for efficient immunoprecipitation. The Halo-Trap consists of an anti-Halo-tag Nanobody conjugated to agarose or magnetic agraose beads. The Halo-Trap combines the Nanobody advantage for immunoprecipitation with the flexibility of the Halo-Tag. You can request a free Halo-Trap sample:
Can I pulldown a HaloTag fusion protein, that is already bound to a substrate?
Yes, you can pulldown a Halo tagged protein, that is already bound to a ligand. The Halo-Trap does not bind to the catalytic center but to a different epitope. Therefore, it is possible to pulldown Halo fusion proteins that are already bound to ligands of the Halo-tag with the Halo-Trap.
How can I elute my HaloTag fusion protein?
After immunoprecipitation with Halo-Trap, one may elute the bound HaloTag fusion protein using either 100 mM citric acid at pH3 or 200 mM glycine at pH 2.5.
What are the binding kinetics of the Halo-Trap?
The binding kinetics of the anti-Halo VHH has been carefully characterized: it is very fast binding and very slow dissociation resulting in a high affinity binding with just 1 nM dissociation constant, which allows for effective pull-downs of low expression proteins.
Binding kinetics:
|
|
KD [M] |
kon(1/Ms) |
kdis(1/s) |
|
Halo-Trap |
2.30.10-9 |
5.40.105 |
1.24.10-3 |
What are excitation & emission wavelengths of HaloTag?
There is no intrinsic excitation and emission in the visible or near infra-red spectrum of the HaloTag protein itself. Excitation and emission depend on the conjugated fluorescent dye. For example Alexa Fluor® 488 has an excitation maximum at 490 nm and an Emission maximum at 525 nm.
Related Content
Immunoprecipitation without additional band
How to conduct a Co-immunoprecipitation (Co-IP)
Which beads should I use for my immunoprecipitation?
Advantages and limitations of different antibody formats in immunoprecipitation
8 Top Tips For Immunoprecipitation
Learn how to save precious hours on your IP, IF, and western blotting experiments
Support
Request a Free Nano-Trap Sample
Nano-Trap system provides fast, reliable, and effective immunoprecipitation of fusion proteins. More than 3,000 peer-reviewed articles have already been published using ChromoTek's Nano-Traps.

