How to immunoprecipitate Flag®-tagged proteins
Flag®-tag (or DYKDDDDK-tag) is a commonly used short peptide tag for multiple applications such as immunoprecipitation (IP), protein purification, immunofluorescence, and Western blotting (WB).
In this blog, we provide an introduction to the IP of Flag®-tagged proteins from cellular extracts. Using our DYKDDDDK Fab-Trap™ as an example, we elaborate on the different steps of IP and highlight the controls that help you achieve the best result in your experiment.
Basic principle of Flag®-IP
IP describes the isolation of a protein from a cellular extract using an antibody, an antibody fragment, or a Nanobody. The Flag®-tag is a short peptide tag and is commonly used as 1x Flag® (amino acid sequence: DYKDDDDK), or 3x Flag® (DYKDHD-G-DYKDHD-I-DYKDDDDK).
It can be cloned to a protein of interest (POI) either N- or C-terminally. A Flag®-tagged protein can be immunoprecipitated from a crude cellular extract using an antibody bound to the Flag®-tag, an anti-Flag® antibody. As Flag® is a registered trademark, anti-Flag® antibodies are often called DYKDDDDK antibodies.
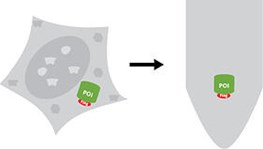
Isolation of a Flag®-tagged protein from a crude sample
What is the DYKDDDDK Fab-Trap™?
To keep the experiment and this blog as simple as possible, we use the DYKDDDDK Fab-Trap™ Agarose as an example for the IP of Flag®-tagged proteins. The DYKDDDDK Fab-Trap™ Agarose consists of an anti-DYKDDDDK antibody Fab-fragment conjugated to agarose beads and is a ready-to-use reagent.
As such, there is no need to discuss the coupling of an anti-DYKDDDDK antibody to protein A or G beads. In addition, the DYKDDDDK Fab-Trap™ saves you experimental and hands-on time.
For more information about the differences in IP using a Fab-fragment or an antibody, please see our blog: Advantages and limitations of different antibody formats in immunoprecipitation

A Flag®-tagged protein (Flag®-tag: red; protein of interest (POI): green) bound by DYKDDDDK Fab-Trap™ Agarose. The DYKDDDDK Fab-Trap™ Agarose consists of an anti-DYKDDDDK antibody Fab-fragment (light and dark blue) conjugated to an agarose bead (gray).
IP of Flag®-tagged proteins from a cellular extract
A typical IP consists of five steps:
- Preparation of cell lysate
- Binding of the antibody to the Flag®-tagged protein
- Washing off unbound molecules
- Elution of the Flag®-tagged protein
- Detection and analysis of the Flag®-tagged protein on SDS-PAGE and Western Blot (WB)
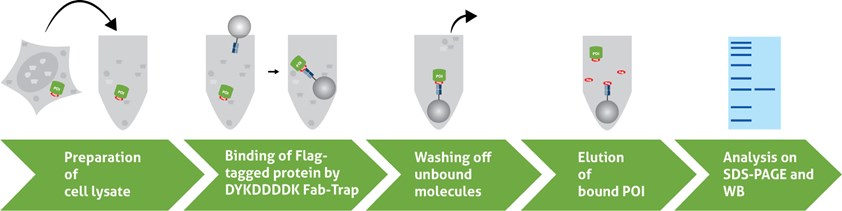
1. Preparation of cell lysate
The preparation of the cell lysate depends on the Flag®-tagged protein and the organism. Normally, about 500 µg (total protein amount) of cell extract is recommended. Here, we focus on mammalian cells, where about ~106-107 cells are needed for a single IP.
2. Binding of the DYKDDDDK Fab-Trap™ to the Flag®-tagged protein
For binding, the DYKDDDDK Fab-Trap™ is incubated with the cell lysate and rotated end-over-end for 1 hour at +4°C. During this incubation step, the DYKDDDDK Fab-Trap™ binds to the Flag®-tagged protein.
3. Washing off unbound molecules
In the following centrifugation steps, the formed DYKDDDDK Fab-Trap™ :Flag®-tagged protein complex sediments at the bottom of the tube. The supernatant containing the unwanted proteins can be discarded and the pellet resuspended. This step should be repeated at least twice.
4. Elution of the Flag®-tagged protein
There are different ways to elute your Flag®-tagged protein from the DYKDDDDK Fab-Trap™. Elution with 2x SDS-sample buffer (Laemmli) is very efficient and is used when the POI is not needed in a native state (e.g., WB). Competitive elution with 3xDYKDDDDK-peptide also works very well and can be used if the Flag®-tagged POI is needed in a native conformation for subsequent analyses.
5. Detection and analysis of the Flag®-tagged protein on SDS-PAGE and WB
To verify the results of your IP, three fractions are usually analyzed on SDS-PAGE and WB: Input, Flow-Through, and Bound/Elution (we recommend the DYKDDDDK tag Polyclonal antibody (Binds to FLAG® tag epitope) from Proteintech for detection because of its high sensitivity).
Analysis of your IP results
In the following, we elaborate on what you should expect to see on your SDS-PAGE/WB and why the analysis of the different fractions is important.
Input
The Input contains the total cell lysate including the Flag®-tagged protein. On SDS-PAGE, there are cellular proteins and the Flag®-tagged protein. On WB, the Flag®-tagged protein can normally be detected with an anti-Flag® antibody. The input fraction is important to verify that your Flag®-tagged protein is expressed and soluble.
Flow-Through
The Flow-Through fraction, also called supernatant, can help you to determine the efficiency of the binding reaction: It contains the unbound proteins of the cell lysate after IP. On SDS-PAGE, you can see cellular proteins, whereas on WB there is ideally no or very little Flag®-tagged protein detectable.
Bound/Elution
The Bound/Elution fraction contains the enriched, purified Flag®-tagged protein. The Flag®-tagged protein can usually be seen on SDS-PAGE and detected on WB.
Elution with SDS-sample buffer is very efficient. The eluate contains the denatured Flag®-tagged protein and the Fab-fragment of the DYKDDDDK Fab-Trap™, which has a size of less than 25 kDa. Please note that an eluate of an IP with a conventional anti-Flag® antibody contains two additional antibody fragments, the heavy and light antibody chains. These may mask the Flag®-tagged protein and/or, if conducting a Co-IP, the interaction partners.
Applying milder elution conditions such as peptide elution with 3xDYKDDDDK-peptide is also efficient. The eluate contains the Flag®-tagged protein only; there are no additional antibody fragments.
![Immunoprecipitation of Flag®-tagged protein from HEK293T cell lysate. During elution with 2x SDS-sample buffer, the enriched Flag® fusion protein and Fab-fragment are released from DYKDDDDK Fab-Trap™. Western blot was probed with DYKDDDDK-tag Polyclonal antibody (Binds to FLAG® tag epitope) (Proteintech, 20543-1-AP) and Nano-Secondary® alpaca anti-human IgG/anti-rabbit IgG, recombinant VHH, Alexa Fluor® 488 [CTK0101, CTK0102] (srbAF488-1). L: Protein marker, I: Input, FT: Flow-Through, B: Bound](/Products/Pictures/Result-ffa.png) |
![Immunoprecipitation of a Flag®-tagged protein with DYKDDDDK Fab-Trap™ from HEK293T cell lysate. During competitive peptide elution, the enriched Flag®-tagged protein is released from DYKDDDDK Fab-Trap™. Western blot was probed with DYKDDDDK-tag Polyclonal antibody (Binds to FLAG® tag epitope) (Proteintech, 20543-1-AP) and Nano-Secondary® alpaca anti-human IgG/anti-rabbit IgG, recombinant VHH, Alexa Fluor® 488 [CTK0101, CTK0102] (srbAF488-1).</em><br /><em>L: Protein marker, I: Input, FT: Flow-Through, E: Elution.](/Products/Pictures/Result-ffa_20211109132909893.png) |
| Immunoprecipitation of Flag®-tagged protein from HEK293T cell lysate. During elution with 2x SDS-sample buffer, the enriched Flag® fusion protein and Fab-fragment are released from DYKDDDDK Fab-Trap™. Western blot was probed with DYKDDDDK-tag Polyclonal antibody (Binds to FLAG® tag epitope) (Proteintech, 20543-1-AP) and Nano-Secondary® alpaca anti-human IgG/anti-rabbit IgG, recombinant VHH, Alexa Fluor® 488 [CTK0101, CTK0102] (srbAF488-1). L: Protein marker, I: Input, FT: Flow-Through, B: Bound. | Immunoprecipitation of a Flag®-tagged protein with DYKDDDDK Fab-Trap™ from HEK293T cell lysate. During competitive peptide elution, the enriched Flag®-tagged protein is released from DYKDDDDK Fab-Trap™. Western blot was probed with DYKDDDDK-tag Polyclonal antibody (Binds to FLAG® tag epitope) (Proteintech, 20543-1-AP) and Nano-Secondary® alpaca anti-human IgG/anti-rabbit IgG, recombinant VHH, Alexa Fluor® 488 [CTK0101, CTK0102] (srbAF488-1). L: Protein marker, I: Input, FT: Flow-Through, E: Elution. |
Controls for Flag®-IP
In addition, controls are very important for the interpretation of IP results and, if necessary, for troubleshooting. As controls, we recommend:
- Positive control: IP of a Flag®-tagged protein that has already been successfully immunoprecipitated, or IP of a multi-tag protein such as GST-3*MYC-6*HIS-3*FLAG-6*HIS-3*HA-6*HIS Fusion Protein from Proteintech.
- Negative control 1: IP of the POI without Flag®-tag. The POI shouldn’t be bound without Flag®-tag and the background should be low. If the POI can be detected in the negative control, it binds non-specifically to the DYKDDDDK Fab-Trap™. This may be prevented by increasing the stringency of the washing step.
- Negative control 2: IP of agarose beads without antibody. Neither the Flag®-tagged POI nor other proteins should be visible in SDS-Page. On WB the Flag®-tagged POI should be detectable in the input fraction only. Otherwise, the Flag®-tagged POI is non-specifically bound to the beads.
As mentioned above, we used the DYKDDDDK Fab-Trap™ Agarose in this blog. DYKDDDDK Fab-Trap™ Agarose binds to the same epitope as Sigma’s Anti-FLAG® M2 Antibody. For more details on the DYKDDDDK Fab-Trap™ Agarose, click here; for more details on the DYKDDDDK-tag Polyclonal antibody (Binds to FLAG® tag epitope), click here.
Test the DYKDDDDK Fab-Trap™ Agarose yourself and request your free sample here:
Free DYKDDDDK Fab-Trap™ sample
Related Content
Flag-tag and 3x Flag-tag: An epitope tag for capture and detection experiments
Immunoprecipitation protocol of DYKDDDDK Fab-Trap™ Agarose
Why is the Fab-Trap™ so Fab-ulous?
Immunoprecipitation without additional band
How to conduct a Co-immunoprecipitation (Co-IP)
Which beads should I use for my immunoprecipitation?
Advantages and limitations of different antibody formats in immunoprecipitation
8 Top Tips For Immunoprecipitation
Learn how to save precious hours on your IP, IF, and western blotting experiments
Support
Request a Free Nano-Trap Sample
Nano-Trap system provides fast, reliable, and effective immunoprecipitation of fusion proteins. More than 3,000 peer-reviewed articles have already been published using ChromoTek's Nano-Traps.
