What is the difference between pyroptosis and apoptosis?
Written by Mehak Passi, PhD Researcher at LMU Munich University
Pyroptosis
Pyroptosis is a type of cell lysis associated with inflammation. Also called regulated necrosis, it is mediated by the catalytic activity of inflammatory caspases (caspase 1 & caspase 5) under certain circumstances). It is characterized by disruption of ionic gradients across the cell membrane and an increase in osmotic pressure which in turn leads to rupture of the cell membrane and release of its contents into the immediate milieu.
Pyroptosis occurs by two mechanisms: canonical and non-canonical pathways. In canonical pathways intracellular sensors such as NLRP1, NLRP3, NLRC4, AIM2, and other inflammasome sensors detect foreign microbial signals and respond by recruiting the adaptor protein ASC, which further recruits pro-caspase 1. Activated caspase-1 cleaves Gasdermin D (GSDMD) to produce GSDMD -NT fragments, generating pores in the plasma membrane bound to the phosphoinositides. In addition, caspase-1 becomes cleaved, producing caspase-1 P10/P20 and P33/ P10 tetramers. These species catalyse the maturation of pro-IL-18 and pro-IL-1β into IL-18 and IL-1β and are then released into the extracellular matrix, causing inflammatory responses.
In the noncanonical pathway, lipopolysaccharides (LPS) from the gram-negative bacterial result in the activation of caspase 4 & caspase 5 (in humans) and caspase 11 (in mice), which further cleave GSDMD and form pores in the plasma membrane. The resulting GSDMD pores allow the release of potassium which further activates the NLRP3 inflammasome and causes IL-1β/ IL-18 maturation. GSDMD pores also release mature cytokines, causing pyroptosis.
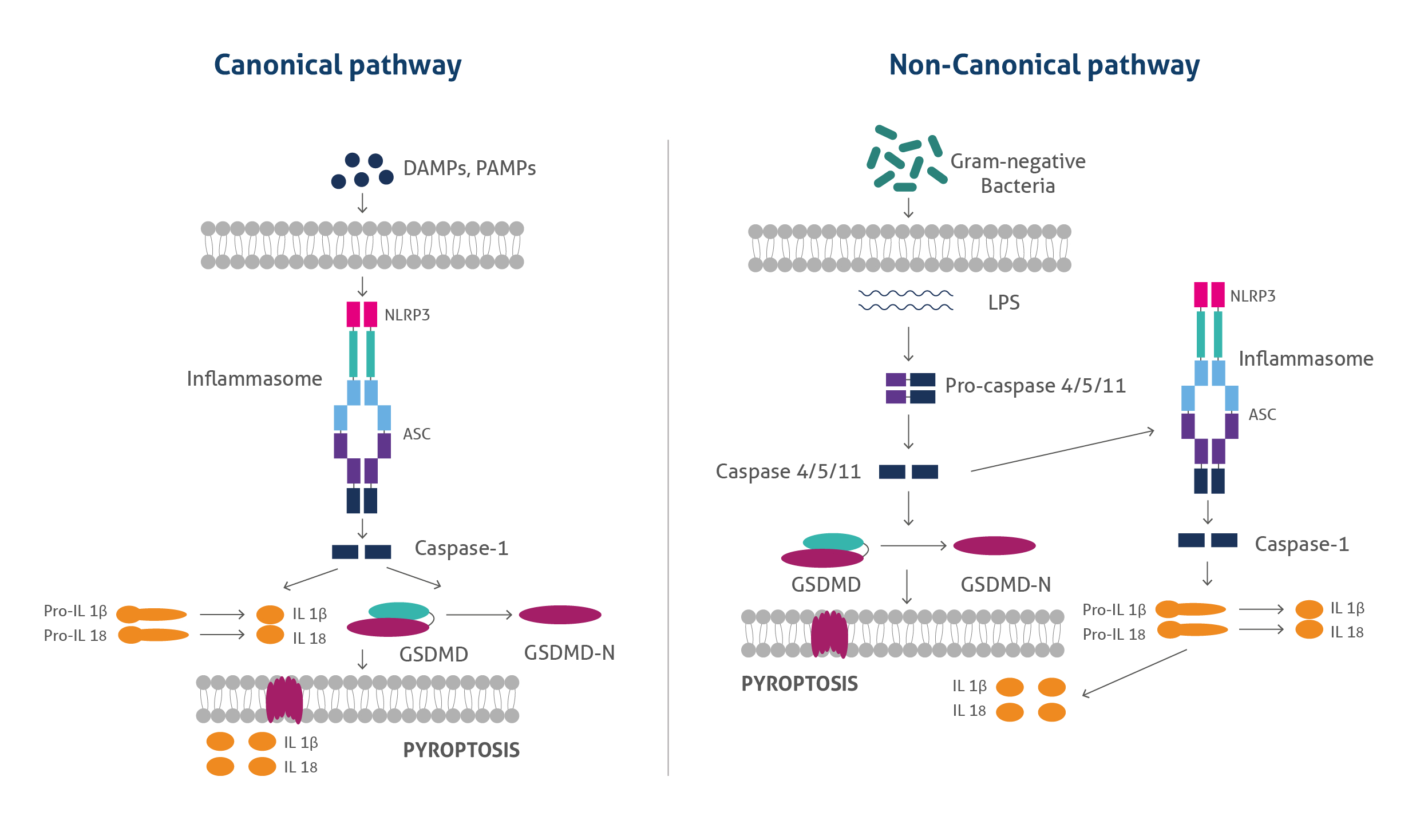
Figure 1: The canonical and noncanonical pyroptosis pathways.
 |
 |
|
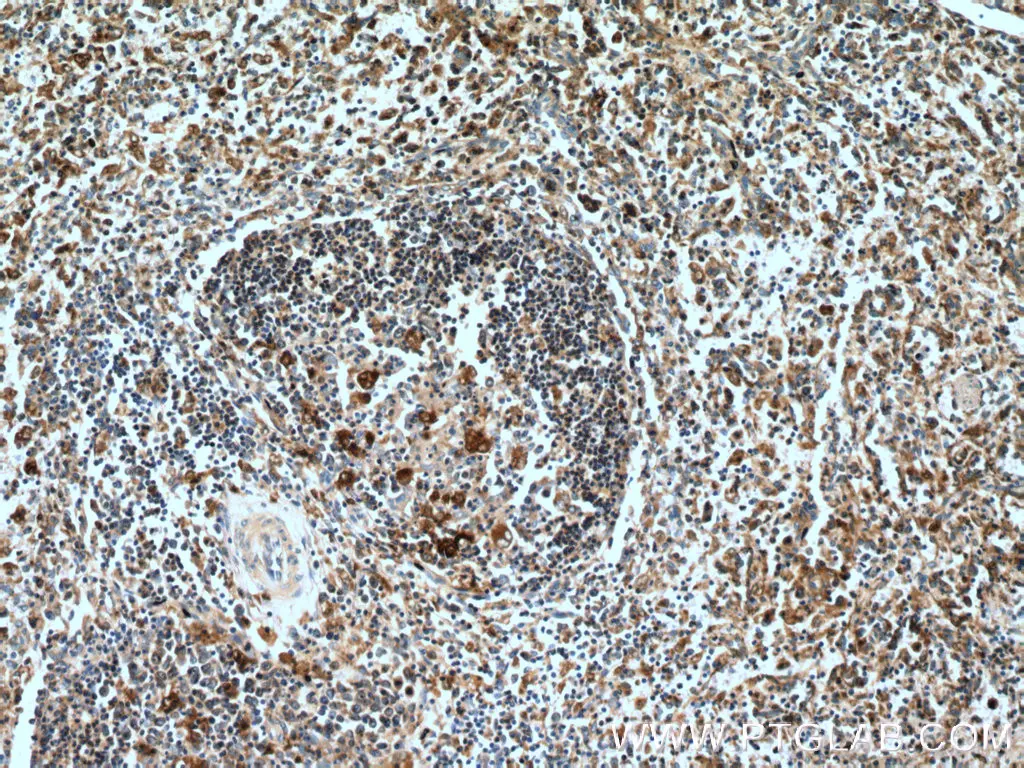
IHC analysis of human spleen tissue using polyclonal caspase 1 antibody (22915-1-AP) at a dilution of 1:200. |
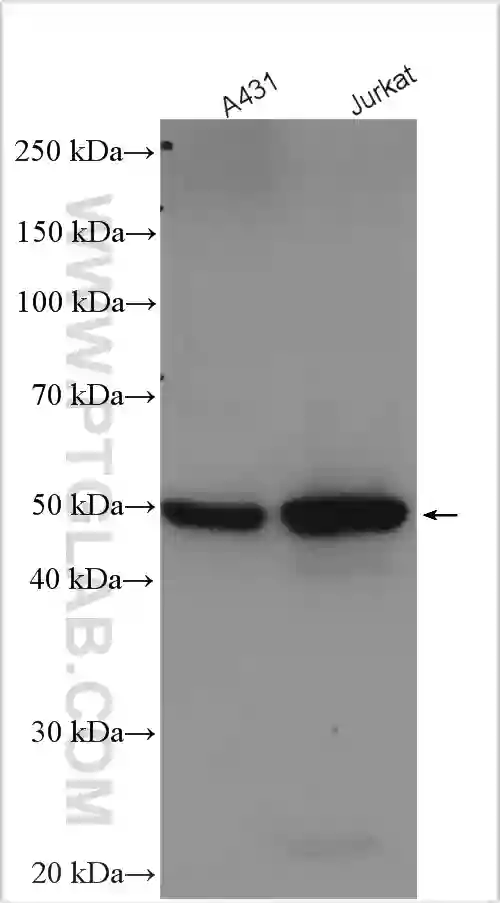
Western blot analysis of various lysates using polyclonal GSDMD antibody (20770-1-AP) at a dilution of 1:5000. |
Inflammatory Aspects of Pyroptosis
Continuous inflammatory responses associated with pyroptosis can lead to increased levels of inflammatory mediators such as IL-1β, IL-18, and HMGB-1, as well as the disruption of host homeostasis. Maintaining this homeostasis plays an important role not only in tumour progression, but also in tumour immunity and immunotherapy. GSDME-mediated pyroptosis promotes tumour cell proliferation through the activation of the ERK1/2 pathway by releasing HMGB1 (High mobility group box 1 protein). The presence of inflammasomes can lead to the activation of caspases 1/4/5/11 and are involved in the pathogenesis of several diseases such as hepatitis, inflammatory bowel disease, arthritis, pneumonia, myocardial infection, and vascular inflammation. Several molecular targets, such as BF-2 and H2O2, have been known to target NLRP3-inflammasome and caspases in arthritis. Pyroptosis also plays an important role in NASH (non-alcoholic steatohepatitis) by activating the GSDMD pathway and canonical pathway.
Further, pyroptosis is closely related to ulcerative colitis (UC) and colitis-associated colon cancer (CAC). In UC, pyroptosis is associated with inflammation caused by IL-1β, IL-18, GSDMD-N, NLRP3 inflammasome, and caspase 1. This process is inhibited by molecules such as Kuijieling (KJL) and dextran sodium sulphate. In CAC, the ERK1/2 pathway is activated by the activation of GSDME, causing pyroptosis and the release of HMGB1, which induces tumour cell proliferation. On the other hand, pyroptosis also occurs in colon cancer cells by activation of caspases 1 and GSDME through Liver X receptor and lobaplatin (an anti-neoplastic agent) signaling.
Pyroptosis also accelerates the development of esophagitis by amplifying inflammatory signals. Esophageal epithelial cells undergo pyroptosis after the activation of caspase 1 and inflammatory cytokines (IL-1β and IL-18). It also plays an eminent role in maintaining health by mediating expression molecules such as GSDMD, extracellular signal-regulated kinase (ERK), signal transducer and activator of transcription 3 (STAT3), and phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) signaling pathways that regulate the cell cycle.
In sum, pyroptosis is akin to a double-edged sword, which on one hand can help the host to clear antigens due to inflammatory effects; but on the other hand, can lead to the development of inflammatory diseases which can cause organ damage. In the context of cancer, the proliferation of cancer cells can be inhibited by inducing cancer cell pyroptosis.
Apoptosis
Apoptosis is a programmed cell death, mediated by specific proteins encoded by the host genome to eliminate single cells from living tissue. It is characterised by plasma membrane blebbing and perturbations, from which small membrane-bound vesicles called apoptotic bodies are released. The execution of apoptosis is carried out by a large family of evolutionarily conserved aspartate-specific cysteine proteases called caspases. Caspases are generally divided into three sub-functional groups: initiator caspases (caspase 2, 8, 9, and 10), executioner caspases (caspase 3, 6, and 7), and inflammatory caspases (caspase 1, 4, 5, 11, and 12). Initiator caspases are responsible for initiating apoptosis, while executioner caspases carry out mass proteolysis leading to apoptosis. Inflammatory caspases are involved in pyroptosis and inflammatory cytokine signaling.
During apoptosis, the plasma membrane undergoes scrambling and lipid organization, mediated by the collective activity of caspases. It is an indispensable physiological mechanism during embryogenesis when tissue formation and regeneration are occurring. Apoptosis is often termed a homeostatic mechanism to maintain cell population in tissues. Apoptosis also functions as a defense mechanism and is a critical process for our immune system to fight infections. It is generally induced by a family of granzyme family molecules produced by activated T cells and NK cells, which further activate caspases and BID (BH3 interacting-domain death agonist). Apart from the granzymes, many pathophysiological conditions and stimuli such as specific receptor molecules (Tumour necrosis factor, Trail, CD95), heat shock, ultraviolet light exposure, cytotoxic drugs, infection from bacteria, toxins, or viruses, H2O2 and ceramide treatment also induce apoptosis. Exposure to irradiation or chemotherapeutic drugs also lead to apoptosis via a p53-dependent pathway.
In general, apoptosis is triggered through two major pathways: intrinsic and extrinsic. Imbalance, dysregulation, or DNA damage triggers the intrinsic pathway by the oligomerisation of B-cell lymphoma-2 (BCL-2) family proteins BAK and BAX. BAK/BAX oligomers permeabilize the outer mitochondrial membrane (MOMP) by forming pores, releasing cytochrome c into the cytosol. The release of cytochrome c leads to the formation of the apoptosome and caspase 3 and 9 activation. Further, these processes trigger activation of other proapoptotic proteins of the BCL-2 family, (PUMA, BAD, BIK, and NOXA) initiating the caspase-processing apoptotic cascade.
The extrinsic pathway is initiated by the activation of cell surface membrane receptors such as Tumour necrosis factor (TNF) and TRAIL receptors (DRS), which oligomerize to form platforms at the cell surface and recruit adaptor proteins such as TNFR1-associated death domain protein (TRADD), Fas-associated death domain (FADD) and receptor-interacting serine/threonine protein kinase 1(RIPK1). This initiates the activation of caspase 8 & 10, forming a death-inducing signalling complex (DISC), and triggering apoptosis. A caspase-like protein termed FLIP present in DISC regulates the activity of caspase 8 & 10, promoting cell survival, cell proliferation, and the production of proinflammatory cytokines. Further ubiquitylation of RIPK1 by cellular inhibitors of apoptosis also regulates the extrinsic apoptotic pathway. Imbalances in this pathway lead to the activation of executioner caspases and trigger programmed cell death. Apoptotic cells further release anti-inflammatory metabolites, shed receptors, and form nucleosomal structures. Phosphatidylserine molecules exposed on the outer surface of the plasma membranes display ‘eat me’ signals to phagocytes.
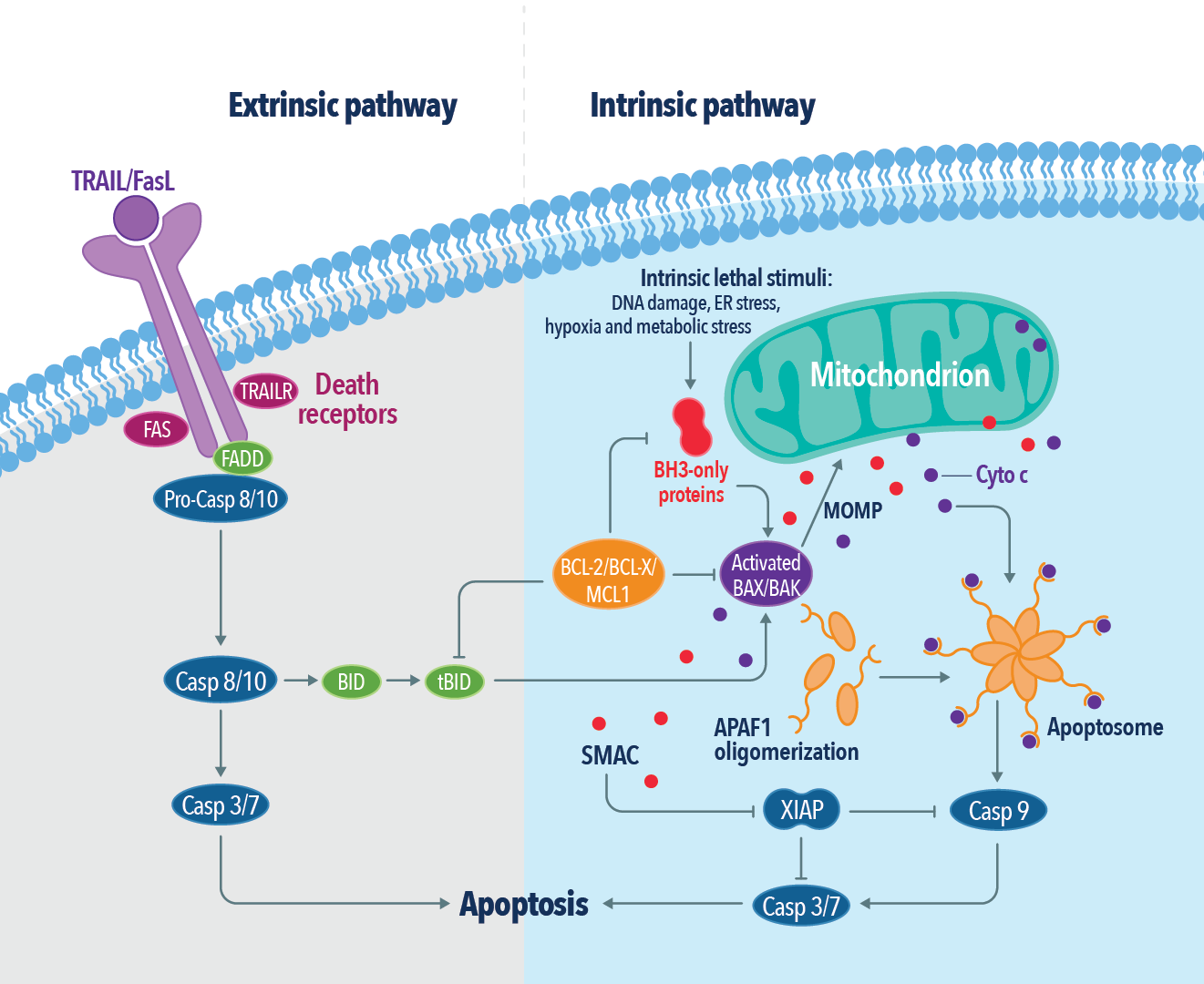
Figure 2: The extrinsic and intrinsic apoptotic pathways.
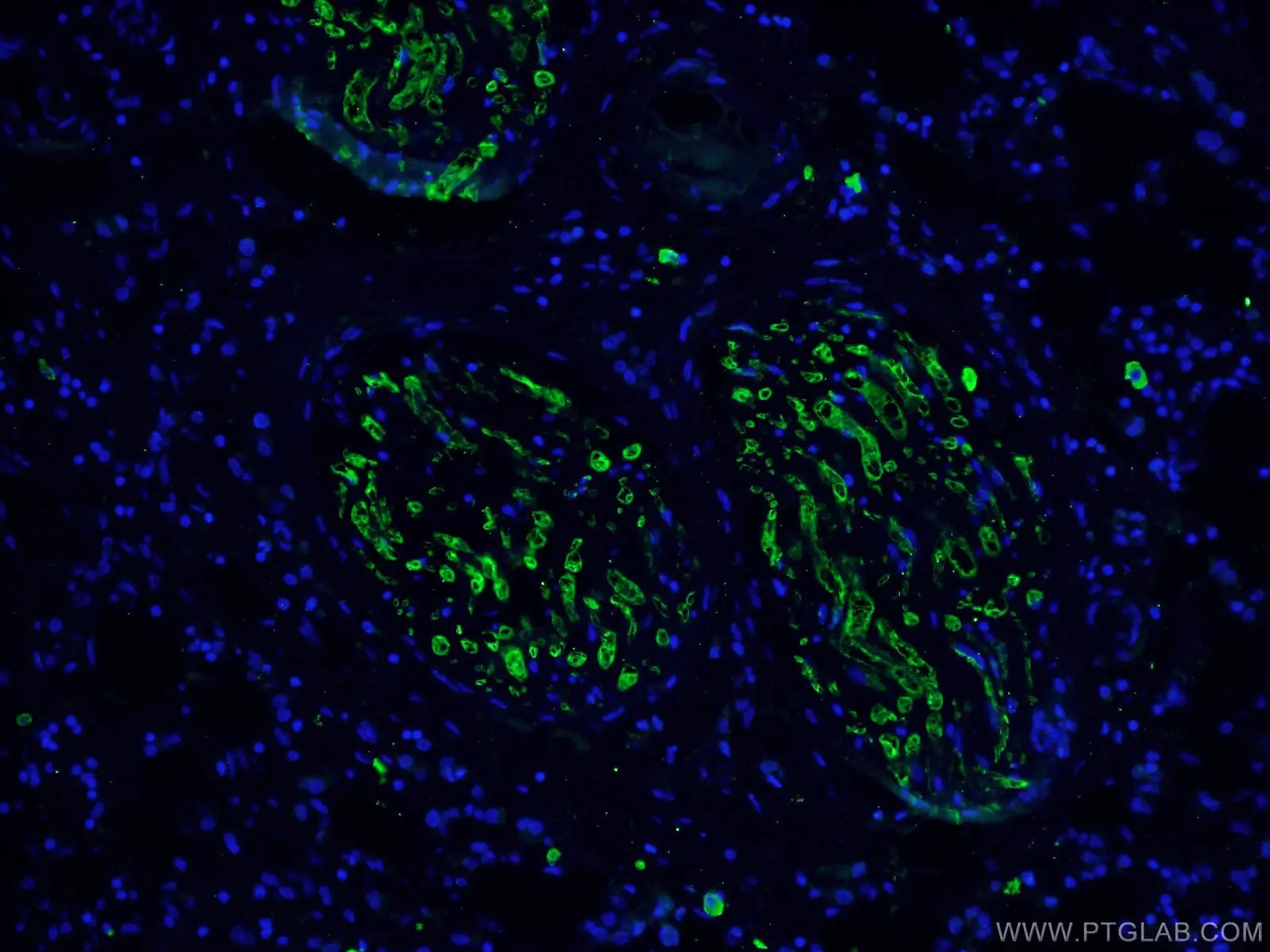 |
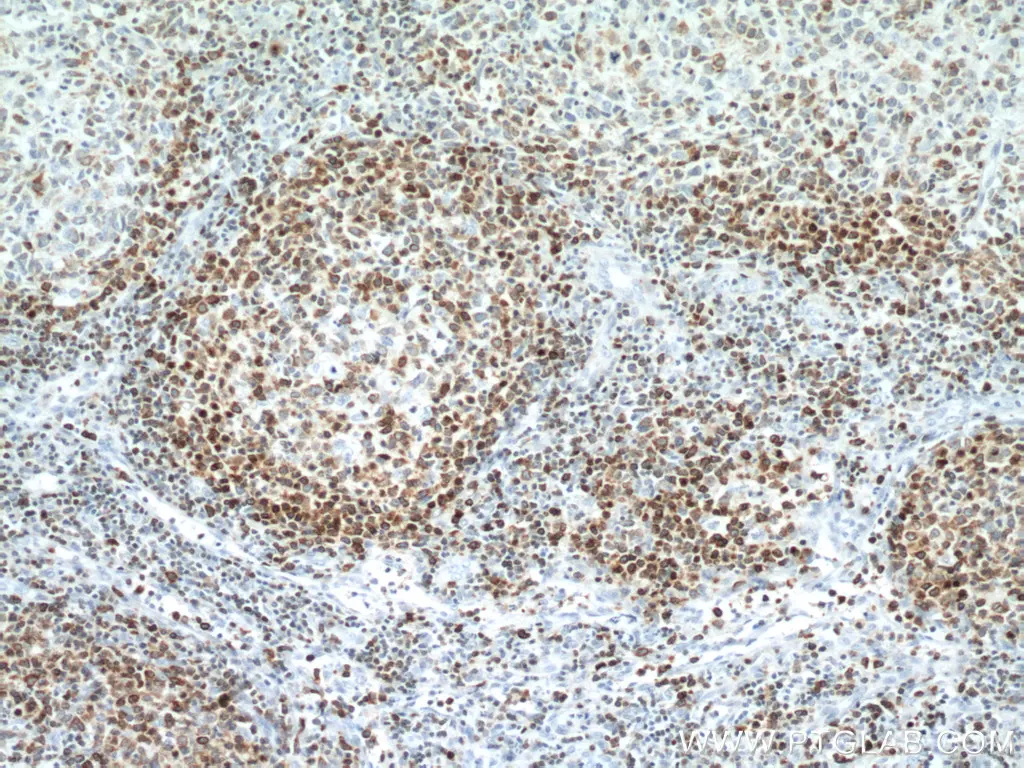 |
|
IF analysis of human breast cancer tissue using monoclonal caspase 3 antibody (66470-2-Ig) at a dilution of 1:100 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Mouse IgG(H+L). |
IHC analysis of human lymphoma tissue using monoclonal BCL2 antibody (60178-1-Ig) at a dilution of 1:12800. |
Table 1: Morphological, mechanistic, and regulatory differences between apoptosis and pyroptosis.
| Apoptosis | Pyroptosis | |
| Morphological Changes |
|
|
| Mechanism |
|
|
| Regulation |
Inhibition of RIPK1 ubiquitination and association of RIPK1 with FADD and procaspase-8 results in apoptotic cell death by caspase-8 activation and subsequent RIPK1 cleavage. |
Regulation occurs through posttranslational modifications in NLRP3 and several other phosphorylation and ubiquitylation events. |
| Mitochondrial involvement | Release of cytochrome c from mitochondria and formation of apoptotic bodies. | Involved in the regulation of Gasdermin D oligomerization and subsequent pore formation in the plasma membrane through the Regulator- Rag complex. |
References
- Necroptosis, pyroptosis and apoptosis: an intricate game of cell death.
- Pyroptosis: host cell death and inflammation.
- Pyroptosis: mechanisms and diseases.
- Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores.
- Cell pyroptosis in health and inflammatory diseases.
- The morphology of apoptosis.
- Apoptosis: a review of programmed cell death.
- Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins.
- Caspases at the crossroads of immune-cell life and death.
- The molecular machinery of regulated cell death.
Related Content
What is the difference between necrosis and apoptosis?
Metabolic regulation of cell death
Caspase cascade antibodies: Caspases play an essential role in apoptosis
How to write a good scientific abstract?
Learn how to save precious hours on your IP, IF, and western blotting experiments
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
