Visualize the onset of metastasis with Chromobodies
The potential of the chromobody technology to study EMT in cell-based screening systems is reviewed by Maier et al., Scientific Reports 2015.
The progression of epithelial cancers into metastatic stages is triggered by a cellular process called “epithelial-mesenchymal transition” (EMT). In response to cytokines, tumor cells shut down the expression of epithelial makers including occludin or E-cadherin and gain mesenchymal markers such as N-cadherin or vimentin. Such cells are losing their cell-cell contacts and apical-basal polarity and become highly migratory and invasive. Considering the importance of this process for cancer progression and formation of metastasis, reliable cellular EMT biomarker and versatile detection systems are necessary to develop novel anti-metastatic therapies.
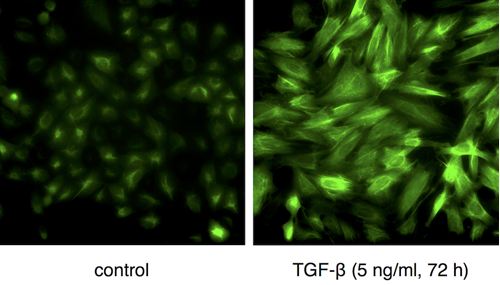
One of the most frequently described biomarker for EMT is the intermediate filament protein vimentin. Elevated levels of vimentin have been correlated with increased formation of metastases of many tumours including prostate, breast cancer or lung cancer. Additionally, recent findings indicate that vimentin plays an active role in reorganising the cellular architecture towards a migratory and invasive phenotype. However, current studies employing vimentin as a biomarker or potential drug target suffer from a lack of appropriate technologies to trace the induction and dynamics of vimentin in cancer relevant cellular model systems.
In the latest issue of Cancer Research published online on September 15th, 2016, Maier et al. reviewed the potential of the chromobody technology to study EMT in cell-based screening systems. They describe a lung cancer cell model stably expressing a Vimentin-Chromobody® based on a single domain antibody (Maier et al., Scientific Reports 2015, 5:13402). This intracellular biosensor visualizes for the first time the expression and reorganization of endogenous vimentin in response to external stimuli. In contrast to conventional fluorescent fusion constructs like GFP-vimentin the chromobody does not affect levels or assembly states of vimentin as an essential EMT biomarker. Thus in combination with high throughput imaging and automated algorithms Maier et al. could not only trace and quantify the induction of EMT by following the chromobody signal but also show the potential of this approach to monitor and screen for the effects of EMT-modulating compounds in real time. In their perspectives the authors point out to their current work focused on the development of 3D spheroid models based on the chromobody technology. The authors claimed that this will greatly expand the possibilities to follow metastatic cells and to screen for compound effects in more realistic tumour-like settings.
Related Content
Nanobody-based reagents by ChromoTek
Intracellular Nanobodies for live-cell analysis in real-time
Chromobodies for live cell imaging
Actin Chromobody for live-cell super-resolution imaging
Visualize Histones in live cells: Histone-Chromobody
Actin Chromobody: Visualization of actin dynamics – NOT alteration
Novel cell cycle modulators identified using the Cell Cycle Chromobody Technology
