Validation Data Gallery
Technical Specifications
| GeneID | 2252 |
| Species | Human |
| Expression | HEK293 |
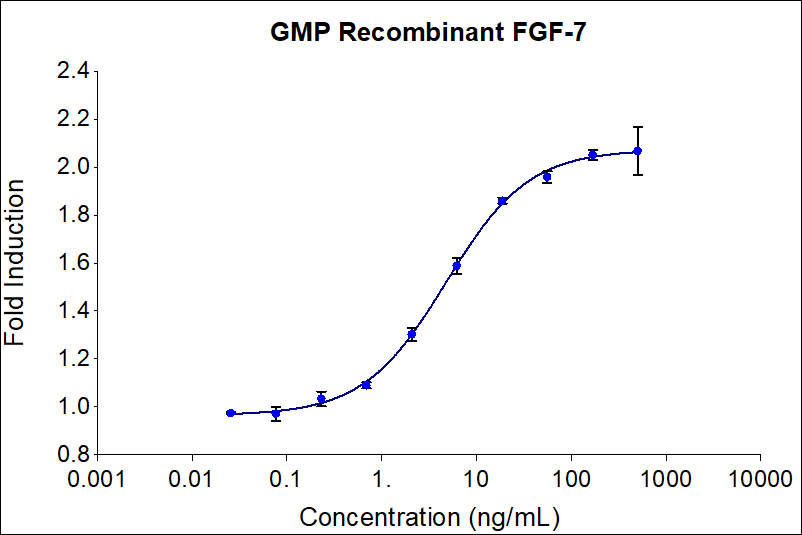
| EC50 | 4-20 ng/mL |
| Specific Activity | minimally 2.00 x 105 IU/mg, typically 3.15 x 105 IU/mg |
| Purity | >95% |
| Endotoxin | <0.1 EU/μg |
| Accession Number | P21781 |
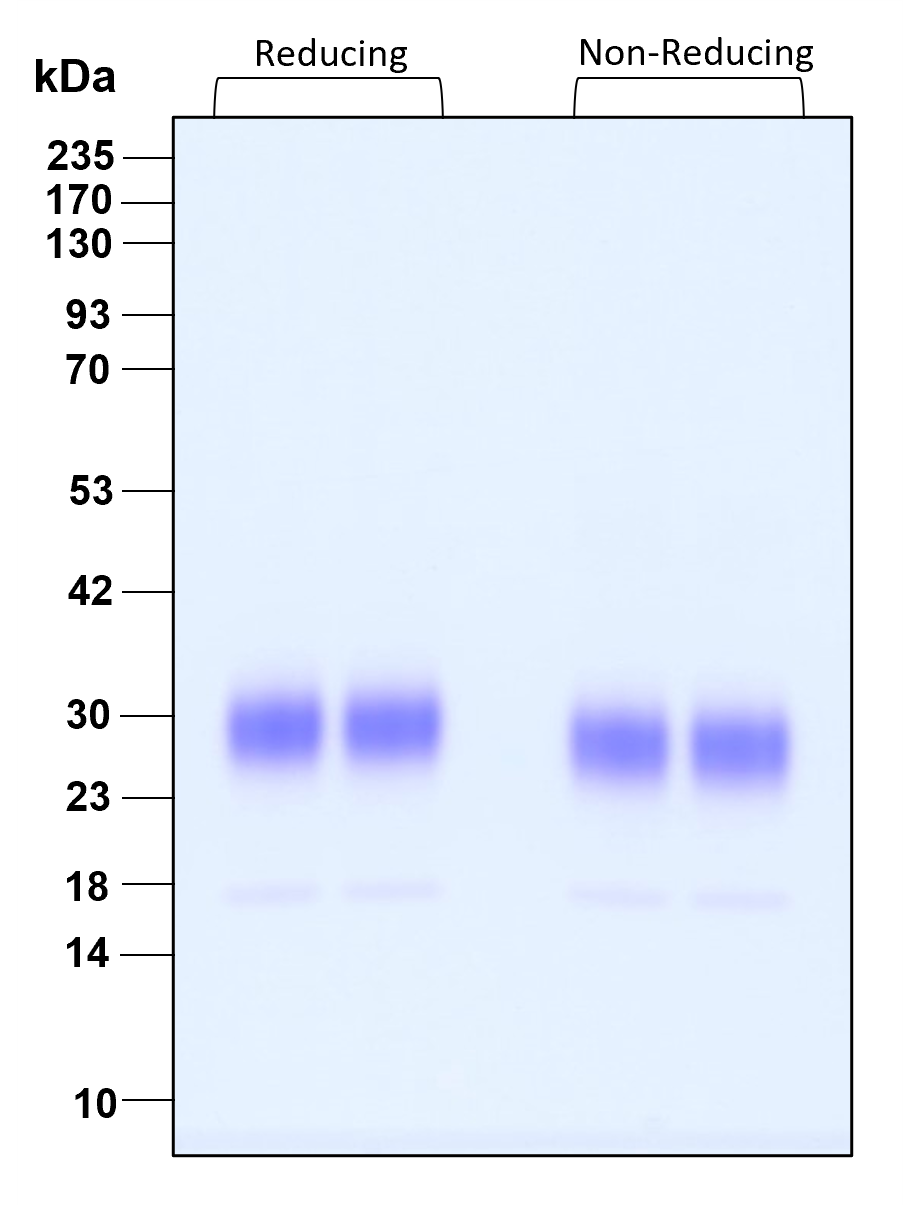
| Molecular Mass | 17 and 25 to 30 kDa reduced, 17 and 24 to 29 kDa non-reduced, monomer, glycosylated |
| Formulation | 1x PBS, See Certificate of Analysis for details |
| Species Reactivity | human,monkey |
Stability and Reconstitution
| Stability and Storage | Product Form | Temperature Conditions | Storage Time (From Date of Receipt) |
|---|---|---|---|
| Lyophilized | -20°C to -80°C | Until Expiry Date | |
| Lyophilized | Room Temperature | 2 weeks | |
| Reconstituted as per CofA | -20°C to -80°C | 6 months | |
| Reconstituted as per CofA | 4°C | 1 week | |
| Avoid repeated freeze-thaw cycles. | |||
| Reconstitution | Briefly centrifuge the vial before opening. It is recommended to reconstitute the protein to 0.2 mg/mL in sterile 1x PBS pH 7.4 containing 0.1% endotoxin-free recombinant human serum albumin (HSA). Gently swirl or tap vial to mix. |
GMP Quality Policies
HumanKine® GMP (Good Manufacturing Practice) recombinant proteins are manufactured and validated in accordance with ISO 13485 quality management system and is compliant with GMP.
Our GMP recombinant proteins are animal component free (ACF), xeno free (XF) and tag free (TF). Read more about these policies here.
Background
N/A
Synonyms
FGF 7, FGF7, FGF-7, Fibroblast growth factor 7, HBGF 7, Keratinocyte growth factor, KGF