The Heart of the Matter: Voltage-Gated Calcium Channels in Cardiovascular Disease
Written by Hannah Joanne Lee, PhD Candidate at Queen Mary University of London
Introduction
Voltage-gated calcium (CaV) channels play a critical role in the regulation of calcium ion (Ca2+) influx into cells, a process vital for the normal function of the heart and blood vessels. Changes in the activity of CaV channels can lead to a range of cardiovascular diseases, including arrhythmias, hypertension, and heart failure. Understanding how these channels contribute to cardiovascular pathology is crucial for developing therapies that can restore normal channel function and improve patient outcomes. This article explores the role of CaV channels in cardiovascular disease mechanisms and treatments which modulate their activity.
What are Voltage-Gated Calcium Channels?
Voltage-gated calcium channels are protein complexes forming a Ca2+ selective pore in the membrane, which is activated by the depolarization of the membrane of excitable cells. CaV channels are ubiquitously expressed in cells such as neurons and muscle cells and are essential for a wide range of physiological events, including neuronal synaptic transmitter release [1], excitation-contraction coupling in the heart, skeletal muscle, and arterial smooth muscle cells [2], and secretion of hormones from endocrine cells [3]. These events are initiated through the transduction of membrane potential changes (electrical signals) into inwards calcium currents, which act as secondary messengers.
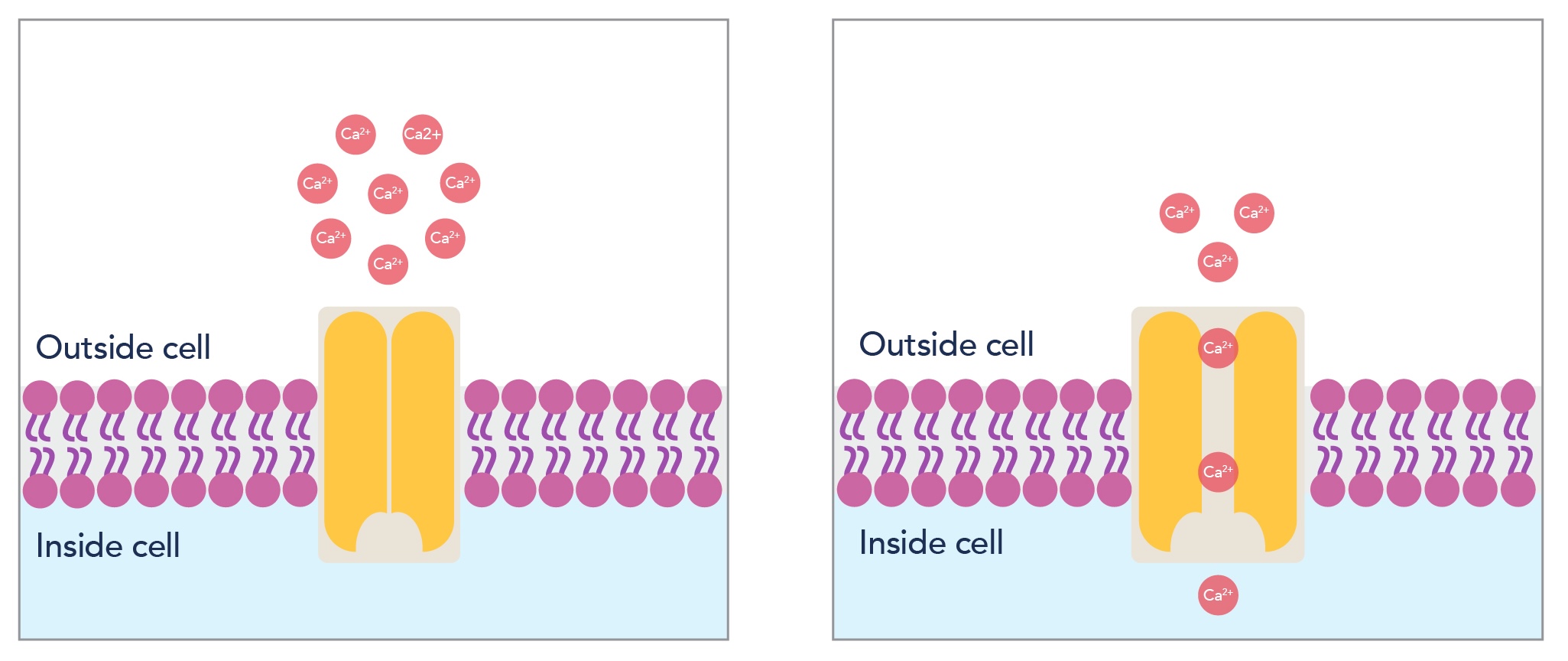
Figure 1: Schematic depicting how voltage-gated calcium channels form a Ca2+ selective pore in cell membranes, allowing diffusion of calcium ions into the cell to trigger physiological events.
Cav Channel Characterization
CaV channels can be categorized into high-voltage activated and low-voltage activated subtypes, and there are several different channel families within these two groups, which are selectively expressed in different tissues and have different pharmacological properties. As shown in Table 1, the CaV channels present in cardiovascular tissue are L-type channels, hence these are the channels mainly involved in cardiovascular disease.
|
Voltage-Activation Type |
Channel Family |
α1 Subunits |
Genes |
Ca2+ Current Type |
Physiological Functions |
|---|---|---|---|---|---|
|
HVA |
CaV1 |
CaV1.1 |
CACNA1S |
L |
Excitation-contraction coupling in skeletal muscle; transcription regulation |
|
CaV1.2 |
CACNA1C |
L |
Excitation-contraction coupling in cardiac muscle and smooth muscle; transcription; regulation; enzyme regulation; transient Ca2+ currents in cell bodies and dendrites |
||
|
CaV1.3 |
CACNA1D |
L |
Cardiac pacemaking; endocrine secretion; auditory transduction; transient Ca2+ currents in cell bodies and dendrites |
||
|
CaV1.4 |
CACNA1F |
L |
Visual transduction (retina) |
||
|
CaV2 |
CaV2.1 |
CACNA1A |
P/Q |
Neurotransmitter release; dendritic Ca2+ transient currents (neurons) |
|
|
CaV2.2 |
CACNA1B |
N |
|||
|
CaV2.3 |
CACNA1E |
R |
|||
|
LVA |
CaV3 |
CaV3.1 |
CACNA1G |
T |
Pacemaking and repetitive firing (neurons) |
|
CaV3.2 |
CACNA1H |
T |
|||
|
CaV3.3 |
CACNA1I |
T |
Table 1: The classification of different types of voltage-gated calcium ion channels and their physiological functions.
Due to their important role in many physiological events, dysfunction and dysregulation of CaV channels has been implicated in a variety of health conditions, including neuropathic pain, cardiac arrhythmia, migraine, hypertension, and epilepsy [4]. Therefore, both the channels themselves and the molecules involved in their regulation are key current and future drug targets for a wide range of conditions.
CaV Channels and Cardiovascular Disease
CaV channels are fundamental for excitation-contraction coupling in the smooth muscle of the heart. In cardiac muscle cells, CaV channels open in response to depolarization, allowing the flow of Ca2+ into the cell. Binding of these Ca2+ ions to ryanodine receptors triggers release of stored Ca2+ from the sarcoplasmic reticulum, increasing the sarcoplasmic calcium concentration further. This is known as calcium-induced calcium release (Figure 2). The Ca2+ ions then act upon tropomyosin complexes, initiating myocyte contraction. This process is tightly controlled, and after each heartbeat, Ca2+ is returned to the sarcoplasmic reticulum via SERCA channels.
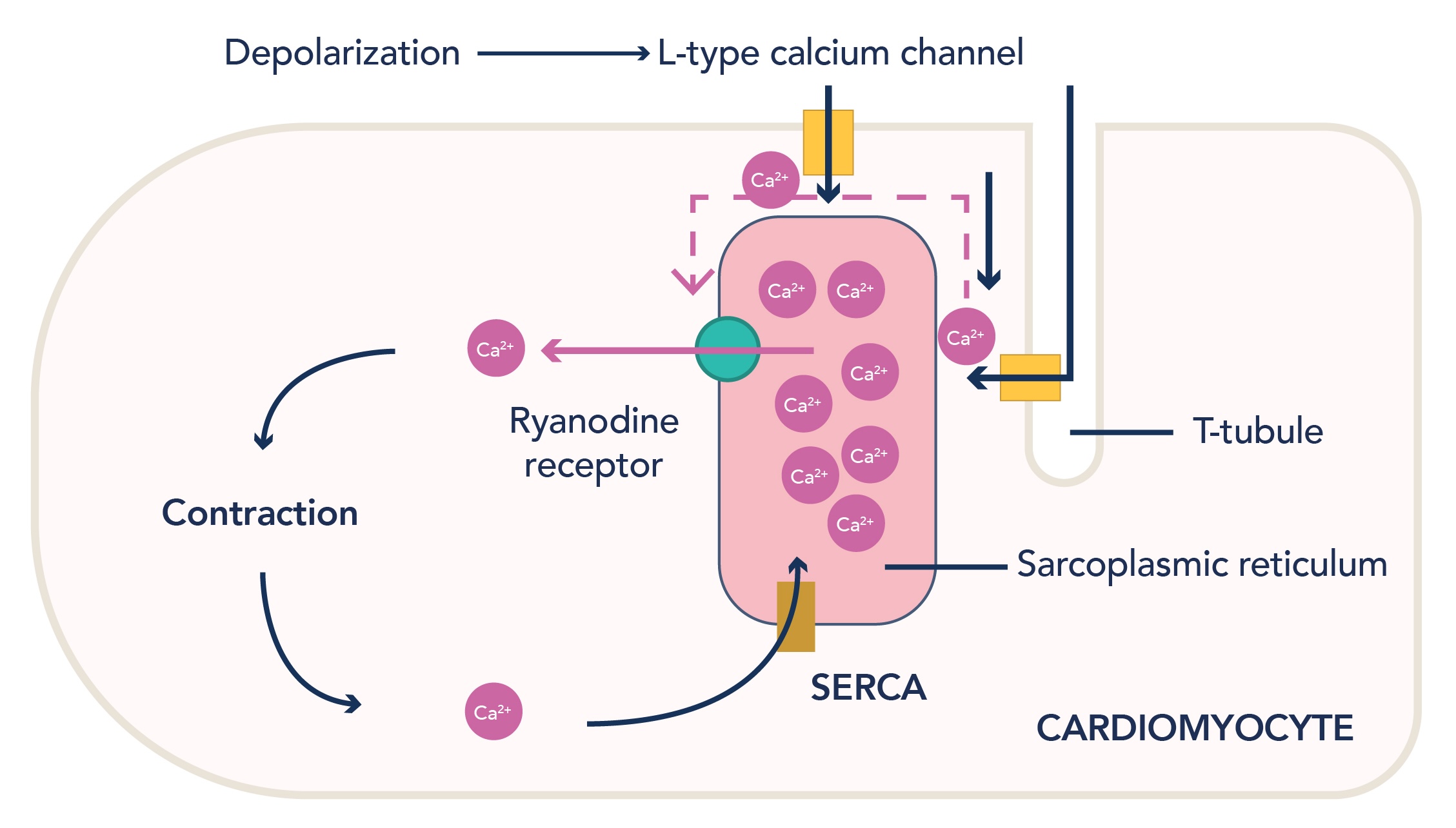
Figure 2: Calcium-induced calcium release in cardiomyocytes. Influx of calcium ions into the cell via L-type channels allows binding of Ca2+ to ryanodine receptors, prompting further release of calcium ions from the sarcoplasmic reticulum into the sarcoplasm, allowing cardiomyocyte contraction.
Changes in CaV channel function can cause disorders such as arrhythmias and hypertension. L-type channels are involved in the ‘plateau’ phase of the cardiac action potential through mediating Ca2+ influx to sustain depolarization. Gain-of-function mutations in CACNA1C (encoding CaV1.2) can lead to Timothy Syndrome, defined by a delay in Cav channel closure leading to delayed cardiac repolarization. Loss-of-function mutations in the same gene can lead to Brugada syndrome, which is characterized by a reduction in Ca2+ influx. Both conditions can cause arrhythmias, ventricular fibrillation, and increase the risk of sudden cardiac death [5].
Interestingly, verapamil, a CaV1.2 blocking drug, is often insufficient to control this condition [6]. The same mutations which cause Timothy Syndrome likely alter the gating and structure of the channel, thus affecting the ability of verapamil to block the channel under these conditions, potentially limiting the use of direct channel blockers in the treatment of pathologies caused by CACNA1C mutations [7]. Beta-blockers, either alone or in conjunction with a CaV channel blocker, are often used to manage the condition by targeting various mechanisms underlying the arrhythmias at once. However, in many cases, a pacemaker is also required to control heart rate [8].
CaV Channels as Therapeutic Targets in Cardiovascular Disease
Calcium channel blockers are widely used to treat cardiovascular diseases. They can be divided into dihydropyridines and non-dihydropyridines [9].
Table 2: Classification of calcium channel blocking drugs used to treat cardiovascular disease.
|
|
CaV Channel Blockers |
Conditions Treated |
|---|---|---|
|
Dihydropyridines |
Amlodipine |
Hypertension; Angina |
|
Felodipine |
Hypertension |
|
|
Isradipine |
Hypertension |
|
|
Nicardipine |
Hypertension; Angina |
|
|
Nifedipine |
Hypertension; Angina |
|
|
Nisoldipine |
Hypertension |
|
|
Non-Dihydropyridines |
Verapamil |
Cardiac Arrhythmias; Hypertension; Angina; Hypertrophic Cardiomyopathy |
|
Diltiazem |
Cardiac Arrhythmias; Hypertension; Angina; Atrial Fibrillation and Flutter |
Dihydropyridines are specific blockers of L-type CaV channels, inhibiting calcium influx into the smooth muscle of the vasculature. This results in vasodilation of blood vessels, reducing blood pressure and hence cardiac afterload, meaning that the heart doesn’t have to work as hard to pump a given amount of blood. Dihydropyridines primarily exert their effect on vascular smooth muscle with limited effects on heart rate and conduction, meaning that they are more commonly used to treat conditions such as high blood pressure and angina [10].
Non-dihydropyridines tend to be more selective for channels in cardiac myocytes, slowing heart rate through reducing Ca2+ entry, thus reducing myocardial contractility, reducing the conduction velocity of the atrioventricular node, and reducing the pacemaker rate of the sinoatrial node [11].
Verapamil is a phenylalkylamine (non-dihydropyridine) calcium channel blocker used in the treatment of a wide variety of cardiovascular disorders. It is selective for cardiac muscle and mainly acts on impulse conduction and ventricular myocytes within the heart. This results in increased coronary and peripheral artery vasodilation and reduced cardiac contraction, heart rate, and atrioventricular conduction [11]. Diltiazem is a non-dihydropyridine calcium channel blocker categorized as a benzothiazepine. It exerts effects in both the heart and blood vessels, with minimal selectivity, but has a similar clinical effect to Verapamil [11]. These drugs both slow heart rate to combat atrial fibrillation and supraventricular tachycardia through reducing Ca2+ entry into cardiac cells, both reducing the force of contraction and slowing electrical conduction through the heart. This also reduces the oxygen demand of the heart, which is useful in the treatment of angina.
Conclusion
CaV channels are crucial regulators of cardiovascular function, playing a pivotal role in excitation-contraction coupling and cardiac rhythm maintenance. Dysregulation of these channels contributes to a plethora of cardiovascular diseases, including arrhythmias, hypertension, and heart failure. Understanding the molecular mechanisms underlying these processes and pathologies has allowed the development of targeted therapies, such as CaV channel blockers, which are currently a first-line treatment for many cardiovascular conditions. Further research into CaV channels may allow for improvement in current therapeutics and the development of novel interventions for treating not only cardiovascular disease but a wide range of pathologies.
Proteintech offers research use antibodies for the study of voltage-gated calcium channels.
|
Target |
Cat No. |
|---|---|
|
CACNA1C |
|
|
CACNA1G |
|
|
Calmodulin 1/2/3 |
|
|
RYR2 |
Learn how Proteintech can support your cardiovascular research here.
Related Content
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

