The role of ferroptosis in human diseases
Written by Dharaniya Sakthivel, PhD student at Baylor College of Medicine
Ferroptosis is a recently discovered form of programmed cell death distinct from apoptosis, necrosis, and autophagy. It is characterized by the accumulation of iron and lipid peroxidation in cells, leading to oxidative damage and the eventual destruction of cellular membranes. Ferroptosis is derived from the Latin word “ferrum,” which means iron.
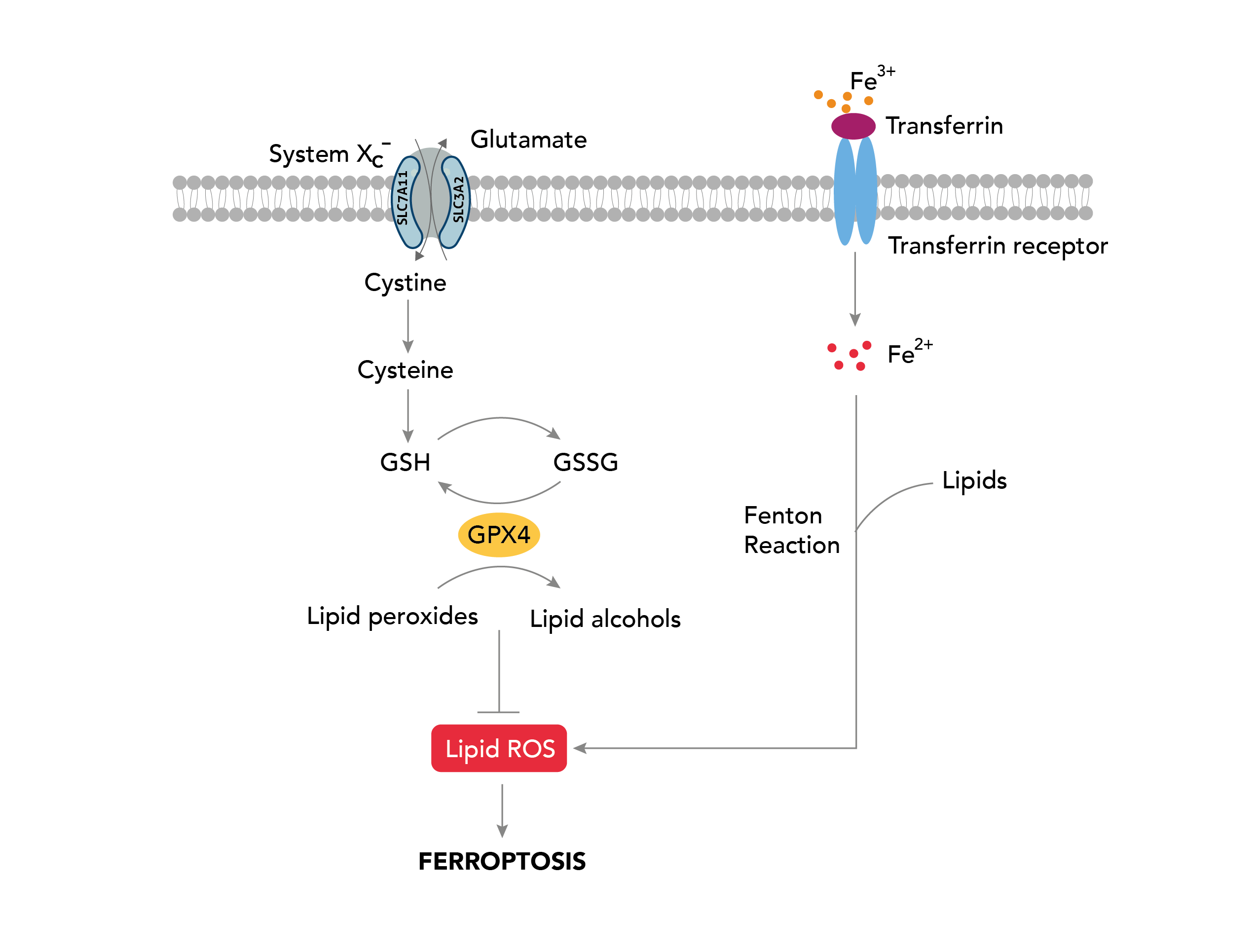
Various cellular stresses, including oxidative stress, metabolic stress, and nutrient deprivation, initiate Ferroptosis. These stresses result in the depletion of cellular antioxidants, such as glutathione. When glutathione levels are low, cells become susceptible to lipid peroxidation, the oxidation of polyunsaturated fatty acid (PUFA) of the membrane lipids [1]. The enzyme lipoxygenase (LOX) catalyzes this process, utilizing iron as an essential cofactor to generate toxic lipid peroxides, such as malondialdehyde and 4-hydroxynonenal [2,3]. The accumulation of these products leads to the production of reactive oxygen species (ROS), triggering cell death. In addition, Ferroptosis is also associated with mitochondrial dysfunction, including changes in mitochondrial membrane potential and alterations in mitochondrial morphology, known to produce ROS, further exacerbating lipid peroxidation [4]. While the physiological roles of Ferroptosis are still being elucidated, it has been implicated in a variety of human diseases as described below.
Proteintech’s Ferroptosis Antibody Kits provide cost-effective tools for studying key proteins involved in the ferroptosis pathway. Perfect for researchers starting a new project, screening multiple prospective targets or those who simply require less volume.
Cancer
One of the most well-studied areas of ferroptosis research is its role in cancer. Ferroptosis has been shown to have both pro-tumor and anti-tumor effects. It can contribute to tumor growth by promoting the death of immune cells that would otherwise impede cancer progression. The increased ROS production during ferroptosis can also contribute to tumor cell proliferation.
On the other hand, cancer cells often have high levels of iron and ROS due to their heightened metabolic activity to sustain uncontrolled cell growth. This makes them particularly susceptible to ferroptosis. Several studies have shown that inducing ferroptosis in cancer cells can be an effective way to selectively kill them without harming the neighboring healthy cells. One promising approach is targeting a protein called glutathione peroxidase 4 (GPX4), which is essential for preventing lipid peroxidation and protecting cells from ferroptosis. Inhibition of GPX4 activates ferroptosis. This approach has shown promise in preclinical studies and is currently being evaluated in clinical trials for various types of cancer [5].
The Cystine/Glutamate antiporter, SLC7A11/xCT, mediates the uptake of extracellular cystine, which is reduced to cysteine, the precursor for glutathione synthesis, protecting cells from oxidative stress [6]. SLC7A11 overexpression is known to confer resistance to ferroptosis in cancer [7]. Strategies targeting SLC7A11 include drugs blocking cystine uptakes such as sulfasalazine, erasin, sorafenib, and HG106. These drugs are labeled as class 1 ferroptosis inducers, and those targeting GPX4 activity are considered class 2 ferroptosis inducers. Both sorafenib and sulfasalazine are FDA-approved drugs that are efficient ferroptosis activators known to inhibit tumor growth. However, due to the off-target effect as a multi-kinase inhibitor [8], therapeutic advances are directed toward targeting metabolic vulnerabilities associated with SLC7A11 pathways in cancer.
One of the holy grails of the cancer field is understanding how the tumor suppressor protein TP53 works to inhibit tumor initiation. Mutant P53 leads to defects in growth arrest, senescence, and apoptosis but can sensitize cells to ferroptosis [9]. It was reported that P53 regulates ferroptosis in part by transcriptionally repressing SLC7A11. However, this is just one piece of the puzzle as to how P53 may regulate lipid peroxides. A multitude of mechanisms by which P53 mediates ferroptosis and its potential in cancers harboring P53 mutation have begun to be recognized.
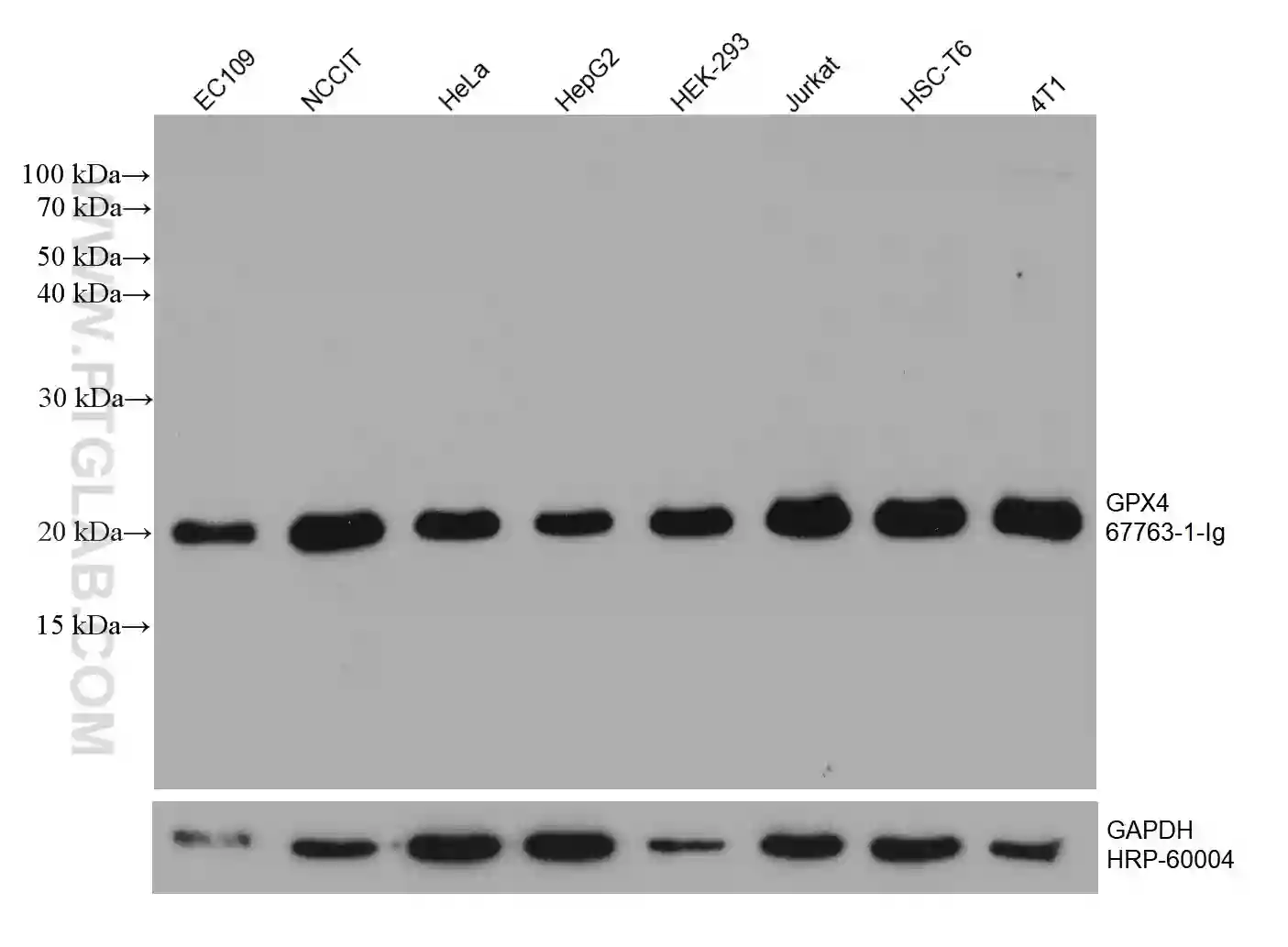
GPX4 Monoclonal Antibody
WB analysis of various cell lysates with GPX4 antibody (67763-1-Ig). |
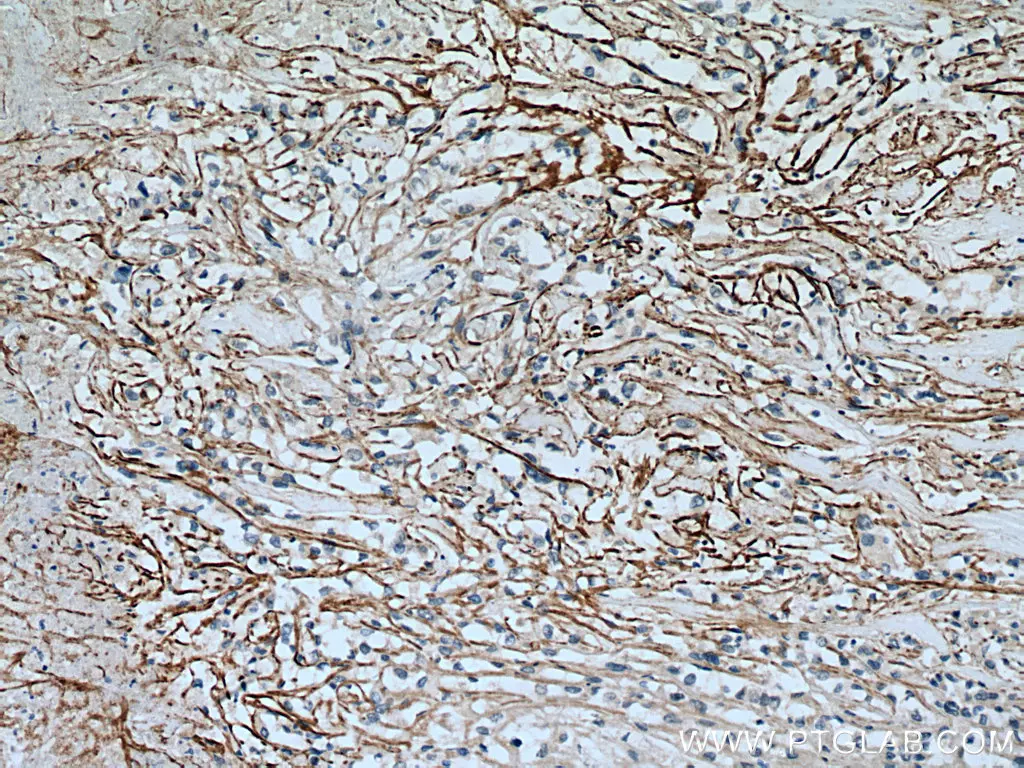
SLC7A11/xCT Polyclonal Antibody
IHC analysis of human renal cell carcinoma with SLC7A11/xCT antibody (26864-1-AP). |
Neurodegenerative disorders
Ferroptosis has been implicated in various neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD).
Increased iron levels and accumulation of lipid peroxidation products have been observed in the hippocampus and cortex of AD patients. In PD, oxidative stress and iron accumulation in dopaminergic neurons have been linked to ferroptosis. Similarly, higher iron accumulation has been seen in the brains of HD patients, and ferroptosis is shown to contribute to neuronal cell death in animal models of HD [10].
Ischemic Injuries
Ischemic-reperfusion (I/R) injuries such as myocardial infarction and stroke are primarily associated with systemic inflammation, a burst of circulating ROS, and organ damage from cell death. While therapeutic methods relating to apoptosis, necrosis, autophagy, and other modes of cell death have been investigated, current treatment options remain challenging [17,18]. Hence, targeting ferroptosis in I/R injury has raised fresh hope. Treatment with ferrostatin-1(Fer-1, a ferroptosis inhibitor) or dexrazoxane (DXZ, an iron chelator) prevents I/R–induced elevations in myocardial enzymes and reduces myocardial infarct size [19]. Inhibition of glutaminolysis, a crucial component of ferroptosis, attenuates I/R-related heart injury [20].
Kidney disease
Ferroptosis has been implicated in acute kidney injury (AKI) and chronic kidney disease (CKD) pathogenesis. In AKI, oxidative stress and lipid peroxidation contribute to renal tubular cell injury and death [11,12]. CKD patients experience iron and lipid deposition, and the induction of ferroptosis in the kidney has been shown to cause renal fibrosis and disease progression [13,14].
Liver disease
Ferroptosis also plays a role in the pathogenesis of liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). In these diseases, there is often an accumulation of iron in the liver, leading to hepatocyte death. Inhibiting ferroptosis protects against liver damage in animal models of NAFLD and ALD [15,16].
Despite the growing evidence of its role in human disease, much remains to be elucidated about ferroptosis and its underlying mechanisms. Researchers are continuing to investigate the key players involved in this form of cell death. As this research progresses, it may become possible to harness the power of ferroptosis to develop new treatments for a wide range of human diseases.
References:
1. Cao, J. Y., & Dixon, S. J. (2016). Mechanisms of ferroptosis. Cellular and Molecular Life Sciences: CMLS, 73(11–12), 2195–2209. doi:10.1007/s00018-016-2194-1
2. Shah, R., Shchepinov, M. S., & Pratt, D. A. (2018). Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Central Science, 4(3), 387–396. doi:10.1021/acscentsci.7b00589
3. Yang, W. S., Kim, K. J., Gaschler, M. M., Patel, M., Shchepinov, M. S., & Stockwell, B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences of the United States of America, 113(34), E4966-75. doi:10.1073/pnas.1603244113
4. Oh, S.-J., Ikeda, M., Ide, T., Hur, K. Y., & Lee, M.-S. (2022). Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discovery, 8(1), 414. doi:10.1038/s41420-022-01199-8
5. Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., … Stockwell, B. R. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell, 156(1–2), 317–331. doi:10.1016/j.cell.2013.12.010
6. Jyotsana, N., Ta, K. T., & DelGiorno, K. E. (2022). The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of cancer. Frontiers in Oncology, 12, 858462. doi:10.3389/fonc.2022.858462
7. Lin, W., Wang, C., Liu, G., Bi, C., Wang, X., Zhou, Q., & Jin, H. (2020). SLC7A11/xCT in cancer: biological functions and therapeutic implications. American Journal of Cancer Research, 10(10), 3106–3126. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33163260
8. Robe, P. A., Martin, D. H., Nguyen-Khac, M. T., Artesi, M., Deprez, M., Albert, A., … Bours, V. (2009). Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer, 9(1), 372. doi:10.1186/1471-2407-9-372
9. Jiang, L., Kon, N., Li, T., Wang, S.-J., Su, T., Hibshoosh, H., … Gu, W. (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature, 520(7545), 57–62. doi:10.1038/nature14344
10. Reichert, C. O., de Freitas, F. A., Sampaio-Silva, J., Rokita-Rosa, L., Barros, P. de L., Levy, D., & Bydlowski, S. P. (2020). Ferroptosis mechanisms involved in neurodegenerative diseases. International Journal of Molecular Sciences, 21(22). doi:10.3390/ijms21228765
11. Linkermann, A., Skouta, R., Himmerkus, N., Mulay, S. R., Dewitz, C., De Zen, F., … Krautwald, S. (2014). Synchronized renal tubular cell death involves ferroptosis. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16836–16841. doi:10.1073/pnas.1415518111
12. Ni, L., Yuan, C., & Wu, X. (2022). Targeting ferroptosis in acute kidney injury. Cell Death & Disease, 13(2), 182. doi:10.1038/s41419-022-04628-9
13. Zhang, Y., Mou, Y., Zhang, J., Suo, C., Zhou, H., Gu, M., … Tan, R. (2022). Therapeutic implications of ferroptosis in renal fibrosis. Frontiers in Molecular Biosciences, 9, 890766. doi:10.3389/fmolb.2022.890766
14. Zhuo, W.-Q., Wen, Y., Luo, H.-J., Luo, Z.-L., & Wang, L. (2022). Mechanisms of ferroptosis in chronic kidney disease. Frontiers in Molecular Biosciences, 9, 975582. doi:10.3389/fmolb.2022.975582
15. Suzuki, Y., Saito, H., Suzuki, M., Hosoki, Y., Sakurai, S., Fujimoto, Y., & Kohgo, Y. (2002). Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcoholism, Clinical and Experimental Research, 26(8 Suppl), 26S-31S. doi:10.1097/01.ALC.0000026830.27338.23
16. Qi, J., Kim, J.-W., Zhou, Z., Lim, C.-W., & Kim, B. (2020). Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. The American Journal of Pathology, 190(1), 68–81. doi:10.1016/j.ajpath.2019.09.011
17. Chen, Y., Fan, H., Wang, S., Tang, G., Zhai, C., & Shen, L. (2021). Ferroptosis: A novel therapeutic target for ischemia-reperfusion injury. Frontiers in Cell and Developmental Biology, 9, 688605. doi:10.3389/fcell.2021.688605
18. Sakthivel, D., Bolívar, B. E., & Bouchier-Hayes, L. (2022). Cellular autophagy, an unbidden effect of caspase inhibition by zVAD-fmk. The FEBS Journal, 289(11), 3097–3100. doi:10.1111/febs.16346
19. Fang, X., Wang, H., Han, D., Xie, E., Yang, X., Wei, J., … Wang, F. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America, 116(7), 2672–2680. doi:10.1073/pnas.1821022116
20. Gao, M., Monian, P., Quadri, N., Ramasamy, R., & Jiang, X. (2015). Glutaminolysis and transferrin regulate ferroptosis. Molecular Cell, 59(2), 298–308. doi:10.1016/j.molcel.2015.06.011
Related Content
What is the difference between pyroptosis and apoptosis
What is the difference between necrosis and apoptosis?
Metabolic Regulation of Cell Death
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.