The power of neutrophils: How to identify the immune system’s first responders
By Maria Alejandra Feliz Norberto, M.S., PhD Candidate at Albert Einstein College of Medicine
What are neutrophils and why should we care?
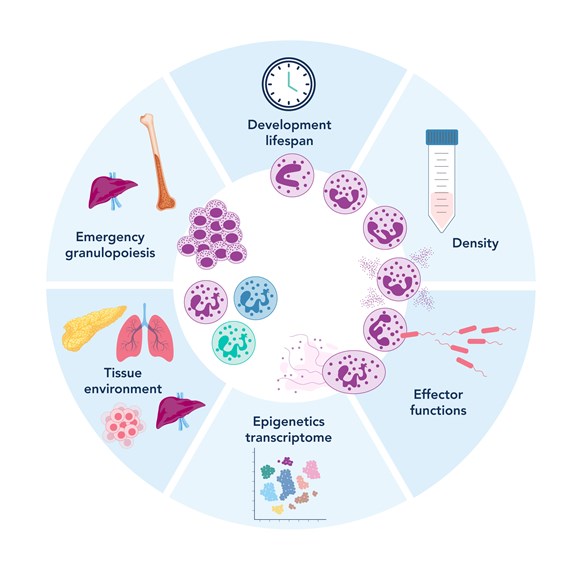
Figure 1. Neutrophil plasticity and heterogeneity (adapted from Gysemans, C., et al, Biomedicines 2025)
Neutrophils are a type of white blood cell, specifically, a form of granulocyte, alongside basophils and eosinophils1,2. They are among the most abundant and fast-acting components of our immune system, making up 40% to 70% of all white blood cells in the human body. Every day, the body produces an astounding 100 to 200 billion neutrophils3.
As the body's first line of defense, neutrophils rapidly respond to infection, tissue injury, and inflammation. Their characteristic multi-lobed nuclei, usually consisting of two to five connected lobes, give them a distinct appearance under the microscope.
Neutrophils originate in bone marrow, where they develop from precursor cells over the course of about a week. Once mature, they enter the bloodstream, constantly patrolling for signs of trouble. Although their lifespan in the blood is short, ranging from 8 to 20 hours in humans, 6 to 12 hours in mice, and up to 3 days in zebrafish, they make up for it with speed and potency4-6. In certain conditions, such as within tumors, neutrophils can survive longer, sometimes persisting for more than five days7.
Whether they are attacking bacteria, neutralizing viruses, or responding to sterile inflammation, neutrophils are built to act fast and act hard. Their efficiency and reactivity are vital to maintaining immune defense, making them a key player in both health and disease.
The role of neutrophils in research
For years, neutrophils were seen primarily as simple foot soldiers of the immune system, essential for fighting infections but not much more. Today, that view has dramatically evolved. We now understand that neutrophils are dynamic and versatile cells, playing key roles not just in immunity, but also in maintaining homeostasis and contributing to various diseases8-14.
In the lab, researchers study neutrophils to uncover their roles beyond acute infections. Their dysfunction is now known to contribute to chronic inflammation, autoimmune diseases, and even cancer. Far from being one-dimensional, neutrophils perform a wide range of functions: they generate reactive oxygen species (ROS), release pro-inflammatory cytokines, form neutrophil extracellular traps (NETs), and participate in tissue remodeling. These actions are essential for orchestrating immune responses, but when poorly regulated, they can become harmful10,15-19. For instance, excessive ROS can damage healthy tissues, and uncontrolled NET formation has been linked to autoimmune disorders such as lupus.
One of the most compelling areas of current research is the role of neutrophils in the tumor microenvironment. Depending on the context, they can either suppress or promote tumor growth. This dual behavior is a hot topic in cancer immunology, as scientists explore how these cells adapt, reprogram, and respond to the unique conditions within tumors.
By studying neutrophil metabolism and function in both health and disease, researchers are uncovering how the immune system adjusts to chronic stress, and how we might one day harness neutrophils to treat a range of conditions.
Tools and methods to study neutrophils
Neutrophils are studied across a variety of model systems, including humans, mice, and zebrafish, each offering distinct advantages for uncovering different aspects of neutrophil biology. Mice are a mainstay in immunology research due to the vast array of genetic tools and disease models available. Zebrafish, with their transparent embryos and transgenic reporter lines, enable high-resolution, real-time imaging of neutrophil development, migration, and behavior in live organisms. Although human samples are more limited in accessibility, they remain essential for studying clinically relevant disease states and validating findings from animal models.
Researchers investigate a wide range of neutrophil functions, including NETosis, reactive oxygen species (ROS) production, chemotactic migration, granule release, and phagocytosis. These functional readouts help us understand how neutrophils contribute to both protective immunity and pathological conditions.
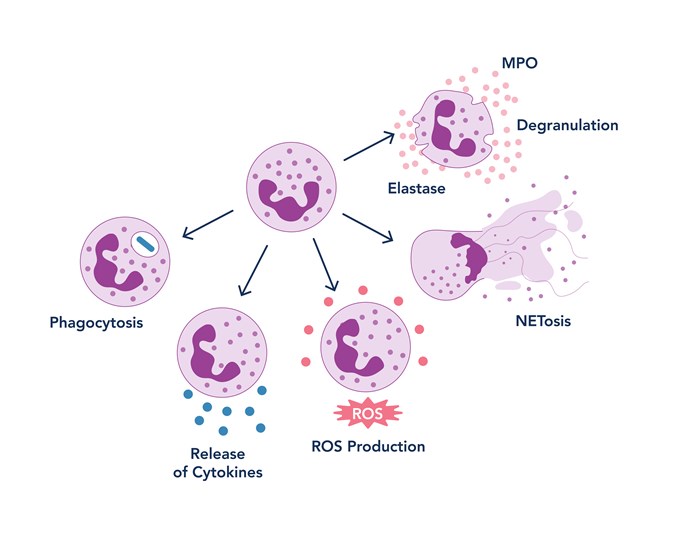
Figure 2. Neutrophil function schema
NETosis is the process by which neutrophils release web-like structures made of DNA and antimicrobial proteins, also known as neutrophil extracellular traps (NETs), to ensnare and kill invading microbes. NETosis can be visualized through live-cell imaging or quantified using flow cytometry. The enzyme peptidylarginine deiminase 4 (PAD4) is a key regulator of this process, driving chromatin decondensation through histone citrullination. Two forms of NETosis are recognized: suicidal NETosis, where the neutrophil dies after releasing its DNA, and vital NETosis, where the cell remains alive and functional even after expelling NETs. To target NETosis, inhibition of PAD4 has been studied, as it represents one of the early steps involved in chromatin decondensation20. Additionally, during chromatin decondensation in NETosis, myeloperoxidase (MPO) and neutrophil elastase (NE) are released21; therefore, researchers also target these enzymes to prevent NET formation.
ROS production is another defining feature of neutrophils. These reactive molecules help kill pathogens but can also contribute to tissue damage when produced in excess. Elevated ROS levels are linked to chronic inflammation, cardiovascular disease, and cancer. Researchers typically measure ROS using fluorescent probes and flow cytometry to assess oxidative stress under different conditions. To target ROS production, NADPH oxidase (NOX2) inhibition is commonly used; for instance, diphenyleneiodonium (DPI) suppresses intracellular ROS generation, while GSK2795039 blocks both intra- and extracellular ROS by specifically inhibiting NOX222-23.
Chemotactic migration is crucial for neutrophils to reach sites of infection or injury. This movement is directed by chemical signals called chemokines and guided by adhesion molecules that help neutrophils exit the bloodstream and navigate through tissues. Impaired or excessive migration is associated with poor immune responses and chronic inflammation. To study this process, scientists often use time-lapse microscopy and transwell assays to track neutrophil movement and directionality.
Granulation involves the release of specialized granules packed with potent antimicrobial enzymes, such as myeloperoxidase (MPO) and neutrophil elastase. These enzymes enhance microbial killing but can also damage host tissues if not tightly regulated. Granule release is commonly measured using biochemical assays and microscopy.
Phagocytosis is one of the most ancient and fundamental immune mechanisms. It is the process by which neutrophils engulf and digest pathogens or cellular debris. In the lab, phagocytosis assays often involve tracking fluorescently labeled particles or bacteria as they are internalized by neutrophils over time.
Identifying neutrophils across model systems
Accurately identifying neutrophils is essential in both basic and translational research, and the approach depends heavily on the model system and the maturity or activation state of the cells. Researchers rely on well-characterized molecular markers and validated antibodies to distinguish neutrophils from other immune cell types.
In zebrafish, commonly used genetic tools include the transgenic lines mpx:GFP24 and lyz:DsRed25, which express fluorescent proteins under the control of neutrophil-specific promoters. These models allow for real-time tracking of neutrophil behavior in live animals and are especially powerful for developmental studies and imaging-based assays.
In humans and mice, neutrophil identification is typically performed using flow cytometry or immunofluorescence-based techniques. Several well-established surface markers help distinguish neutrophils from other leukocytes, as well as assess their developmental stage and activation status.
For human neutrophils, key markers include:
-
CD66b, CD15, CD16, and CD11b: common surface antigens used to label mature neutrophils in peripheral blood.
-
CD62L (L-selectin) and CXCR4: useful for determining activation state and migratory behavior.
-
CD45: a general leukocyte marker expressed across all white blood cells.
For mouse neutrophils, important markers include:
-
Ly6G, CD11b, and Gr-1: used in combination to identify and isolate mature neutrophils.
-
CXCR4, CD62L, and c-Kit: provide insight into cell maturity and tissue localization.
-
CD45: serves as a pan-leukocyte marker in mouse models as well.
Here is a table summarizing key markers across species:
|
Stage |
Human Markers |
Mouse Markers |
Zebrafish Tools |
|
Progenitor |
c-Kit+, Sca-1+ |
Not applicable |
|
|
Mature Neutrophil |
mpx:GFP, lyz:DsRed |
||
|
Activated |
Activity-based reporters |
||
|
General Leukocyte |
CD45+ |
CD45+ |
Pan-leukocyte staining |
Combining these markers with functional assays helps researchers not only identify neutrophils but also understand their role in health and disease.
References:
-
Gungabeesoon, J., et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 186(7):1448-1464.e20 (2023).
-
Ng, L. G., Ostuni, R. & Hidalgo, A. Heterogeneity of neutrophils. Rev. Immunol. 19, 255–265 (2019).
-
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
-
Lu, R.J., et al.Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex- and age-related functional regulation. Nat Aging. 1, 715–733 (2021).
-
Dixon, G., et al. A method for the in vivo measurement of zebrafish tissue neutrophil lifespan. ISRN Hematol. 2012, 915868 (2012).
-
Lahoz-Beneytez, J., et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 127, 3431–3438 (2016).
-
Ng, M. S. F., et al. Deterministic reprogramming of neutrophils within tumors. Science. 383, eadf6493 (2024).
-
Soehnlein, O. & Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Rev. Immunol. 10, 427–439 (2010).
-
Ley, K., et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Rev. Immunol. 7, 678–689 (2007).
-
Nordenfelt, P. & Tapper, H. Phagosome dynamics during phagocytosis by neutrophils. Leukoc. Biol. 90, 271–284 (2011).
-
Day, R. B. & Link, D. C. Regulation of neutrophil trafficking from the bone marrow. Mol. Life Sci. 69, 1415–1423 (2012).
-
Tu, H., et al. Dying to defend: neutrophil death pathways and their implications in immunity. Sci. (Weinh). 11, e2306457 (2024).
-
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
-
Dixon, S. J., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149, 1060–1072 (2012).
-
Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303, 1532–1535 (2004).
-
Kim, H. K., et al. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. 108, 812–820 (2006).
-
Bagaitkar, J., et al. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood. 131, 2367–2378 (2018).
-
Baz, A. A., et al. Neutrophil extracellular traps in bacterial infections and evasion strategies. Immunol. 15, 1357967 (2024).
-
Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Med. 23, 279–287 (2017).
-
Lewis, H.D., et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 11, 189–191 (2015).
-
Papayannopoulos, V., et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 191, 677–691 (2010).
-
Buck, A., et al. DPI selectively inhibits intracellular NADPH oxidase activity in human neutrophils. 3, 488–497 (2019).
-
Hirano, K., et al. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal. 23, 358–374 (2015).
-
Elks, P.M., et al. Measuring inflammatory cell migration in the zebrafish. Methods Mol Biol. 769, 261–75 (2011).
-
Li, L., et al. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 287(30), 25353–60 (2012).
Related Content
Hematopoietic Stem and Progenitor Cells (HSPCs): What They Are and How to Identify Them | Proteinte…
Endothelial Cells: Gatekeepers in HIV Disease Progression | Proteintech Group
Guide to Flow Cytometry Panel Building | Proteintech Group
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.