The FlexAble way to improve your immunofluorescence with IBEX
Written by Aanandita Kothurkar and Natalia Jaroszynska from UCL Institute of Ophthalmology
Immunofluorescence in zebrafish: basic principles and challenges
Zebrafish are commonly used animal models in research. They offer many experimental advantages, such as being relatively inexpensive to house, having a high fecundity, and being simple to genetically manipulate. Moreover, zebrafish larvae are ideal for microscopy due to their optical transparency and tools available for depigmentation of the less transparent tissues, such as the eye.
Immunofluorescence (IF) is a widely used technique that allows you to label proteins of interest using antibody-based detection and fluorescence microscopy to visualize cells and subcellular structures of interest. Standard IF uses primary antibodies that detect specific proteins or antigens, and these are visualized using secondary antibodies tagged with fluorophores. Read the blog here for expert tips on maximizing your IF in zebrafish and other tissues.
An obstacle when using standard immunostaining approaches is the limited availability of host species for antibody detection. Only one antibody raised in a particular species can be used on the same tissue because fluorescently conjugated secondary antibodies broadly detect primary antibodies raised in the same host. This cross-reactivity limits the number of proteins that can be visualized simultaneously using conventional immunostaining techniques. This is further exacerbated in zebrafish, where there are limited antibodies for specific antigens, and a vast majority of them are raised in rabbits.
So, what can be done to overcome this issue?
Directly conjugated antibodies
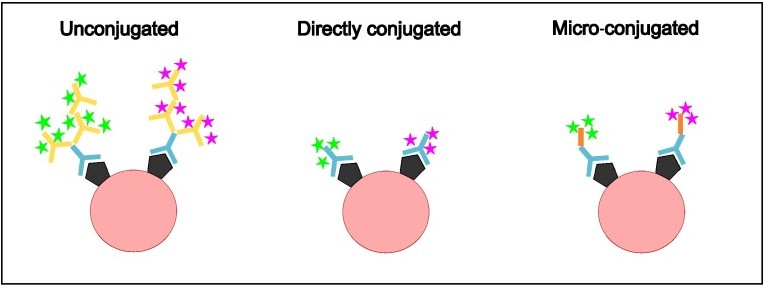
Figure 1. Comparison of methods of antibody conjugation. Unconjugated primary antibodies (indicated in blue) bind to the antigen (shown in black) and are, in turn, bound by secondary antibodies which fluoresce (indicated by yellow, with fluorescence shown as stars) specific to the host in which the primary antibody was raised. Directly conjugated antibodies are primary antibodies already bound to a fluorophore and bind to the antigen. Micro-conjugated antibodies link the primary antibodies to the fluorophores using a linker protein (indicated in orange).
An obvious solution to the problem is using conjugated antibodies. These are primary antibodies that are directly conjugated to a fluorophore of interest, removing the need for secondary antibody-based detection (Fig. 1). These allow one to simultaneously visualize multiple proteins with antibodies raised in the same species and reduce the immunofluorescence protocol to a quick, single step staining. Conjugated antibodies are commercially available but may not cover all targets or might have limited conjugates that don’t fit in your experiment. Therefore, Proteintech offers FlexAble antibody labeling kits that allow you to conjugate your favorite primary antibodies in your own lab!
FlexAble kits
Unlike other conjugation kits, FlexAble kits don’t require a buffer exchange and can be used with as little as 0.5µg of primary antibody, allowing you to conjugate the validated antibodies that you already have in the lab. The kits use a high-affinity linker to conjugate your primary antibody to a fluorophore of your choice from a range of options in a quick, 10-minute process (Fig.2). This allows you to combine multiple antibodies raised in the same host in one tissue (Fig.3) and conjugate the same antibody with different fluorophores, rather than conjugating your entire antibody aliquot to one fluorophore. This flexibility is important when it comes to combining immunofluorescence with transgene-labeled proteins, which are restricted to a particular fluorophore.
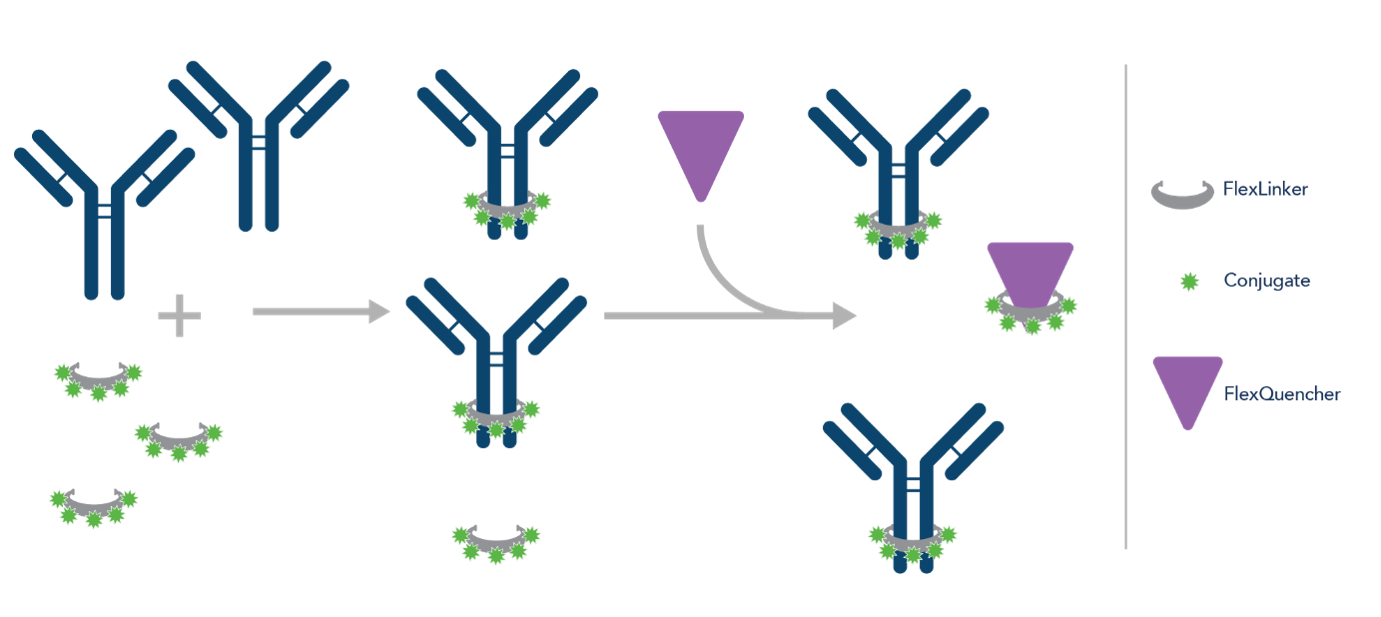
Figure 2. Schematic showing how FlexAble works.
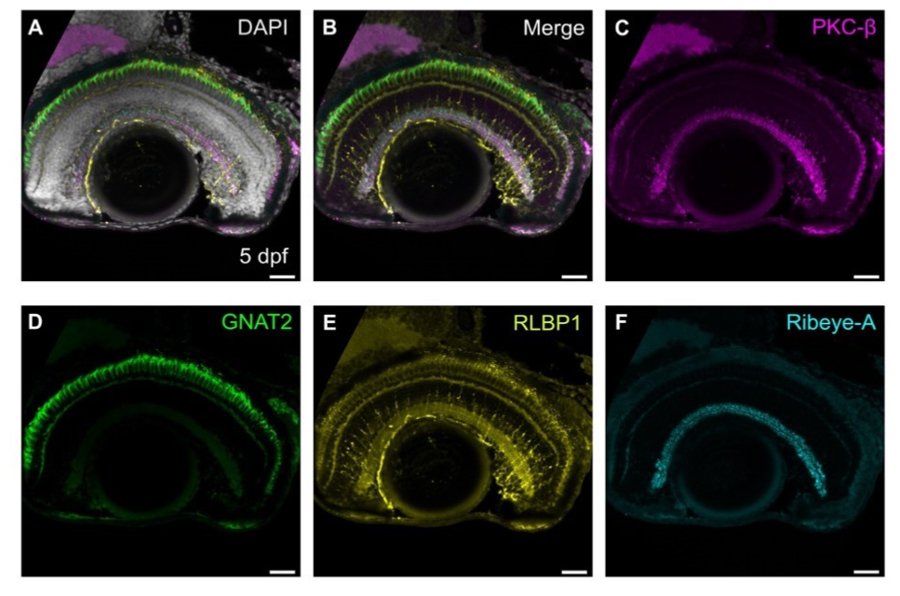
Figure 3. Four different antibodies, each raised in rabbit, can be visualized on the same piece of tissue using FlexAble micro-conjugation. Epifluorescence image of sagittal section of 5 days post fertilization (dpf) retina, immunolabeled with PKC-ß (magenta, bipolar cells), GNAT2 (green, cones), RLBP1 (yellow, Müller glia), Ribeye-A (cyan, ribbon synapses). Scale bars - 25µm. Image from Kothurkar et al. 2024.
The FlexAble kits are also compatible with non-Proteintech antibodies, making them a fantastic and versatile tool for anyone. Further, they are compatible with different microscopes, so no fancy technology is required to adapt these into your lab!
Expert Tip: Conjugated antibodies do not amplify the signal of the primary antibody, as traditional polyclonal antibodies do. This may mean that the fluorescence from lowly expressed proteins may be dimmer with conjugated antibodies and hence will need to be individually tested.
Utilizing FlexAble kits for multiplexing: IBEX
FlexAble conjugation kits not only allow us to simultaneously view multiple antibodies raised in the same host but can also be applied to more advanced IF techniques. If you work with rare or precious tissue, such as human samples from patients, it is important to maximize the information you can gain from the limited tissue that is available. Also, your biological question may require you to visualize large numbers of different proteins or cell types simultaneously within the same tissue, which cannot be done with standard IF approaches due to the limited number of channels available on a fluorescence microscope. Iterative Bleaching Extends Multiplexity (IBEX) could be the perfect solution to overcome this issue.
What is IBEX and how does it work?
IBEX is a multiplexed immunolabeling technique that allows you to visualize up to 60 antibodies on the same piece of tissue (Radtke et al., 2020). Sequential rounds of immunolabeling and imaging are performed, and after each round, the antibody fluorescence is bleached using a lithium borohydride solution. This quenches the fluorescence and allows you to stain the sample with a new set of conjugated antibodies. Once all the images have been acquired, open source software SimpleITK (Radtke et al., 2022) is used to align these images based on a common channel that does not bleach, such as the nuclear stain DAPI (Fig. 4). This allows you to superimpose the different rounds of staining and view all your antibodies together in silico (Fig.5). Our lab has optimized this approach for the zebrafish retina; however, it can be applied to any tissue in which immunofluorescence is possible.
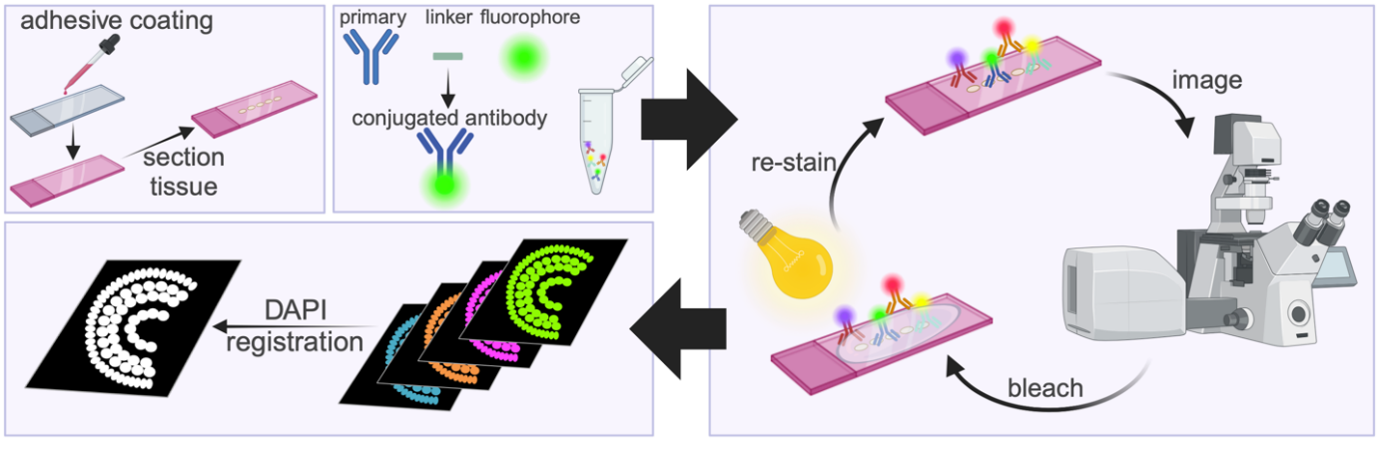
Figure 4. Schematic of the IBEX method. Slides are coated with chrome alum gelatin to prevent tissue loss, then tissue sectioned onto slides. Antibodies are micro-conjugated by mixing the primary antibody with a linker and fluorophore. The antibodies are applied to the slide, incubated, imaged, then bleached using bright light and lithium borohydride before being re-stained. After the imaging rounds are completed, nuclear stains (DAPI) are used to register the image, allowing for all stains to be visualized together. Made with Biorender. (Kothurkar et al., 2024)

Figure 5. IBEX enables simultaneous labeling of all retinal cell types. (A) Merge of confocal images showing a single sagittal retinal section immunolabeled with 11 different markers: Ribeye-A (dark blue), carbonic anhydrase (CA1, purple), glutamine synthetase (GS, red), PKC-β (green), Zrf-1 (cyan), HuC/D (orange), GFP transgene (yellow), calbindin (CalB, magenta), peripherin-2 (PRPH2, light blue), GNAT2 (orange), and RLBP1 (pink) and the merge separated into different rounds of staining. Scale bars - 25µm (Kothurkar et al., 2024).
Essentials for IBEX:
There are several essential components you need for IBEX. These are summarized below and in detail in our recent preprint (Radtke et al., 2020; Kothurkar et al., 2024).
- Chrome Alum gelatin: Thinly coating the slide with chrome alum gelatin prior to cryosectioning helps the tissue adhere better to the slide and reduces tissue loss over multiple rounds of immunolabeling.
- Fluoromount G mounting medium: This mounting medium is water soluble and forms a semi-permanent seal that allows you to remove the coverslip from the slide between rounds of imaging by simply immersing the slide in PBS. (Expert tip: Minimize the time your slide is mounted with the coverslip, as this makes it quicker and less damaging to the tissue when removing the coverslip).
-Lithium borohydride: This bleaching solution is faster acting than previous methods of fluorophore quenching (Expert Tip: Most efficient when bleaching solution is applied alongside exposure to bright light.)
Optimizing micro-conjugation & IBEX for your antibodies
The first round of IBEX is the only round where secondary antibodies can be used; hence, it can be performed over 2 days like in traditional immunostaining. You can then bleach these and use conjugated antibodies from round 2 onwards. While we have found FlexAble kits to be ideal for this, various other fluorophores like AlexaFluor or Atto are also compatible with IBEX. Check out the IBEX knowledgebase (Yaniv et al., 2024) to see what antibodies other researchers have tested with IBEX!
Further, some antibodies against proteins that are highly expressed can be difficult to bleach and must hence be used in the last round. There is a degree of optimization required to test the compatibility of your antibodies with the FlexAble kits and to design your IBEX panels and the order in which the antibodies are applied. To read more about how we optimized this process for the zebrafish, Turquoise Killifish and Xenopus retina, refer to our preprint.
Combining IBEX with other methods in the zebrafish tool kit
Often, a reliable antibody may not be available for your gene of interest, particularly if you work with non-mammalian models, such as the zebrafish. In these situations, gene expression can be visualized using fluorescent in situ hybridization (FISH) methods. Aside from multiplexing antibodies, our lab has combined IBEX with RNA detection methods such as Hybridization chain reaction (HCR) (modified FISH) (Choi et al., 2018). As HCR uses AlexaFluor/Atto fluorophores, they can be bleached using the bleaching methods mentioned above. Since mRNA staining patterns can be difficult to interpret and determine which cells they are expressed by, the HCR-stained images can be overlaid with antibody staining to correlate mRNA expression with cell type to identify the expression pattern of your gene (Fig. 6).
Furthermore, the obvious advantage of zebrafish is that many tissues can be imaged in wholemount, without the need for tissue sectioning. Indeed, in our latest preprint, we optimized IBEX and showed that it can be further combined with wholemount IF in zebrafish (Fig. 7) with slight modifications to the protocol, such as variation in the incubation and bleaching times (details in preprint).
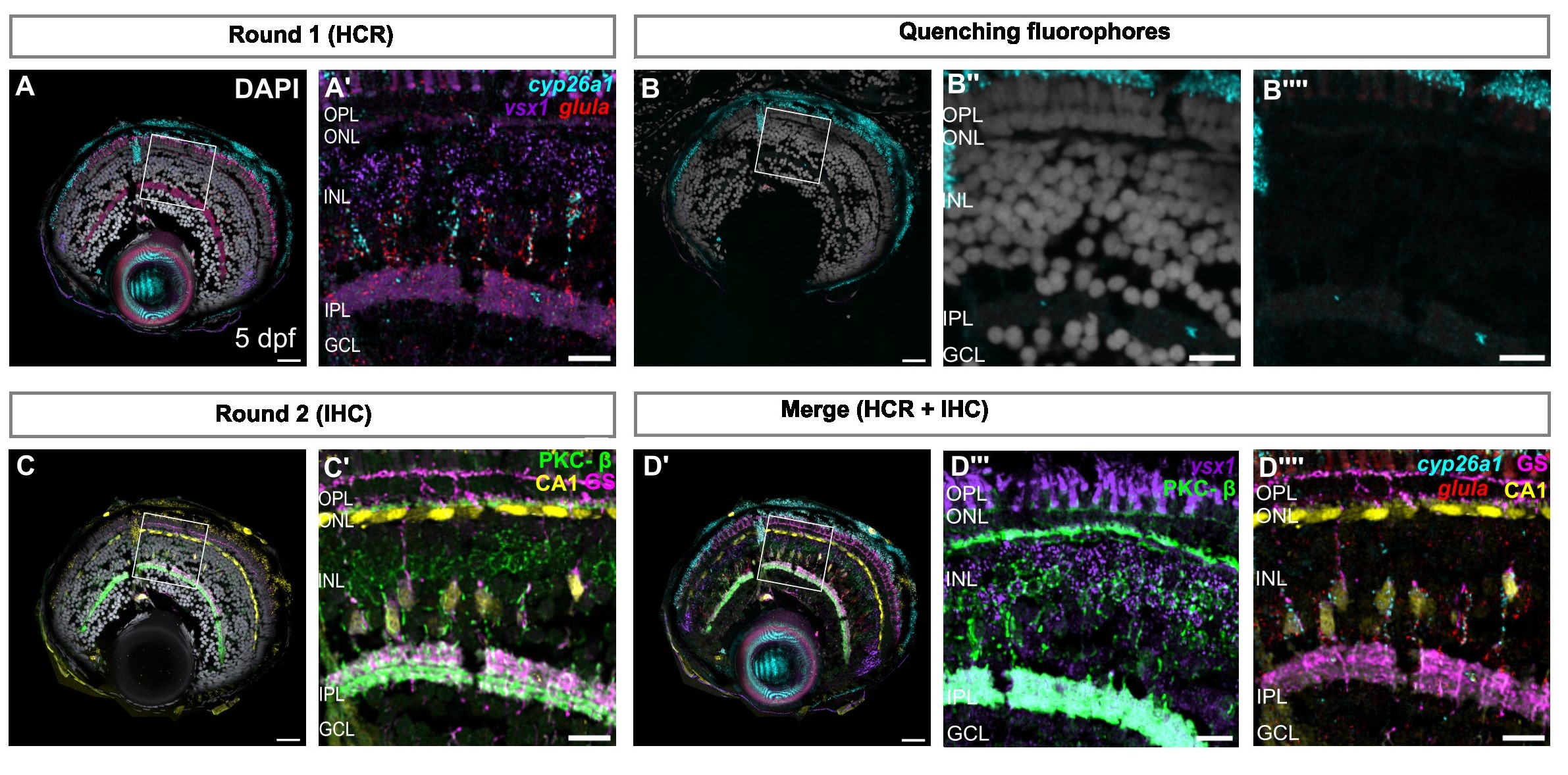
Figure 6. (A) Confocal images of retinal sections showing mRNA expression of cyp26a1, glula, and vsx1, using in situ hybridization chain reaction (HCR). (A′) Zoom on the region of interest indicated in (A). (B-B′′′’) Confocal images showing reduced signal of AlexaFluor-488, 555, & 647, after heating in sodium citrate at 60ºC. (C) Confocal images of retinal sections immunolabeled with CA1 (yellow), PKC-β (green), and GS (magenta). (C′) Zoom on region of interest indicated in (C). (D) SimpleITK registered image, showing overlay of both rounds of imaging and overlay of in situ probes and antibodies detecting Müller glia and bipolar cells, respectively. (D′′-D′′′) Zoom of region of interest shown in (D, D′). Scale bars - 25µm for whole retina, 10µm for zoom images (Kothurkar et al., 2024).
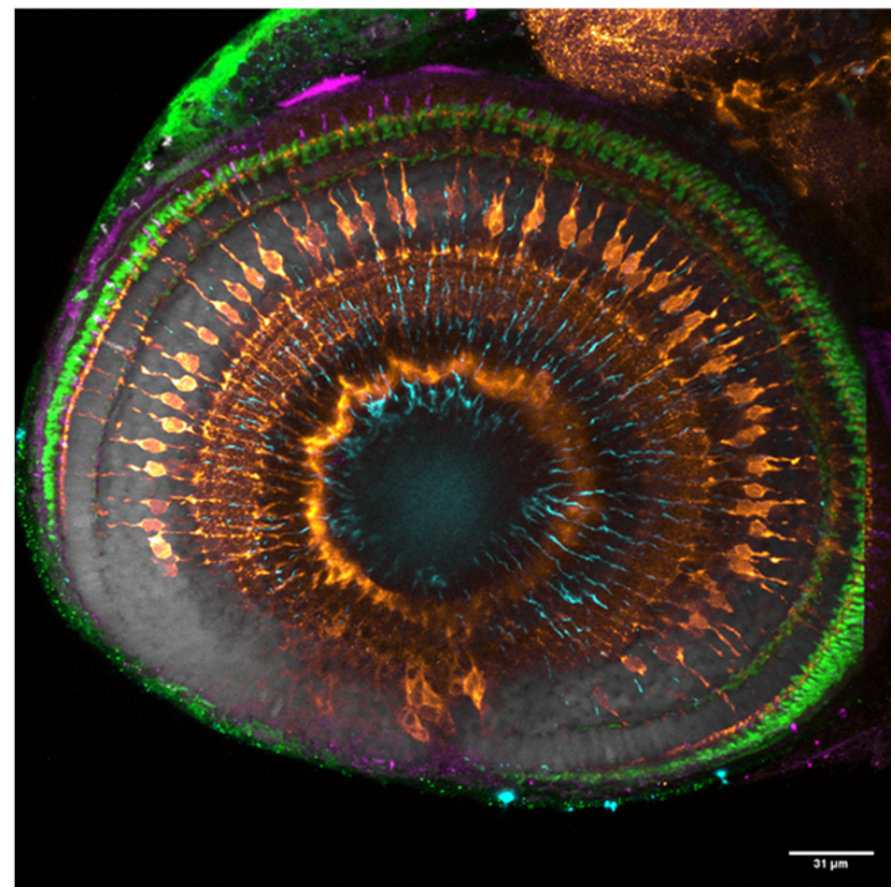
Figure 7: Merge of wholemount confocal images of immunolabeling to detect Zrf-1 (cyan), GNAT2 (green), GS (orange), and blue opsin (magenta) with DAPI (grey). Scale bars - 25µm (Kothurkar et al., 2024).
IBEX is a powerful technique that can revolutionize how we study interactions between multiple cell types, which is important to understand how tissues as a whole are altered during development, regeneration, or degeneration. The FlexAble kits from Proteintech offer an easy and convenient way to conjugate your primary antibodies, increasing the number of markers you can use in a single-step or multiplexed immunofluorescence experiment. Our preprint covers our optimization of these kits for zebrafish, as well as non-conventional animal models like the Turquoise Killifish and Xenopus!
Proteintech offers a wide range of FlexAble and FlexAble 2.0 kits which have been optimized for immunofluorescence workflows. Find the right kit for your experiment.
References
Choi, H.M.T. et al. (2018) ‘Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust’, Development, 145(12), p. dev165753. Available at: https://doi.org/10.1242/dev.165753.
Kothurkar, A. et al. (2024) ‘Iterative Bleaching Extends Multiplexity (IBEX) imaging facilitates simultaneous identification of all cell types in the vertebrate retina’, bioRxiv, p. 2024.02.28.582563. Available at: https://doi.org/10.1101/2024.02.28.582563.
Radtke, A.J. et al. (2020) ‘IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues’, Proceedings of the National Academy of Sciences, 117(52), pp. 33455–33465. Available at: https://doi.org/10.1073/pnas.2018488117.
Radtke, A.J. et al. (2022) ‘IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues’, Nature Protocols, 17(2), pp. 378–401. Available at: https://doi.org/10.1038/s41596-021-00644-9.
Yaniv, Z. et al. (2024) ‘Iterative Bleaching Extends Multiplexity (IBEX) Knowledge-Base’. Zenodo. Available at: https://doi.org/10.5281/zenodo.13122300.
Related Content
Immunofluorescence techniques in zebrafish retina
FlexAble Antibody Labeling Kits
GFP-Booster: GFP Nanobody for better images in immunofluorescence
A Guide to Immunostaining the Cerebellum
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

