Spectral Compensation in Flow Cytometry
Excerpt from Mastering Flow Cytometry: Best Practices and Tips webinar given by Dr. Shweta Puntambekar, Ph.D. This webinar excerpt has been edited for clarity.
What is spectral compensation?
Excerpt from Mastering Flow Cytometry: Best Practices and Tips webinar given by Dr. Shweta Puntambekar, Ph.D. on April 11th, 2023. This webinar excerpt has been edited for clarity.
What is spectral overlap?
As with any technique that uses lasers, suppose I have this experiment that I’ve stained with something that fluoresces in FITC and something that fluoresces in PE. I have a FITC-specific laser that excites just FITC and emits a FITC-specific spectrum, and then I have a PE-specific laser that excites just PE that emits a PE-specific spectrum. It is these emission spectra that will result in your data.
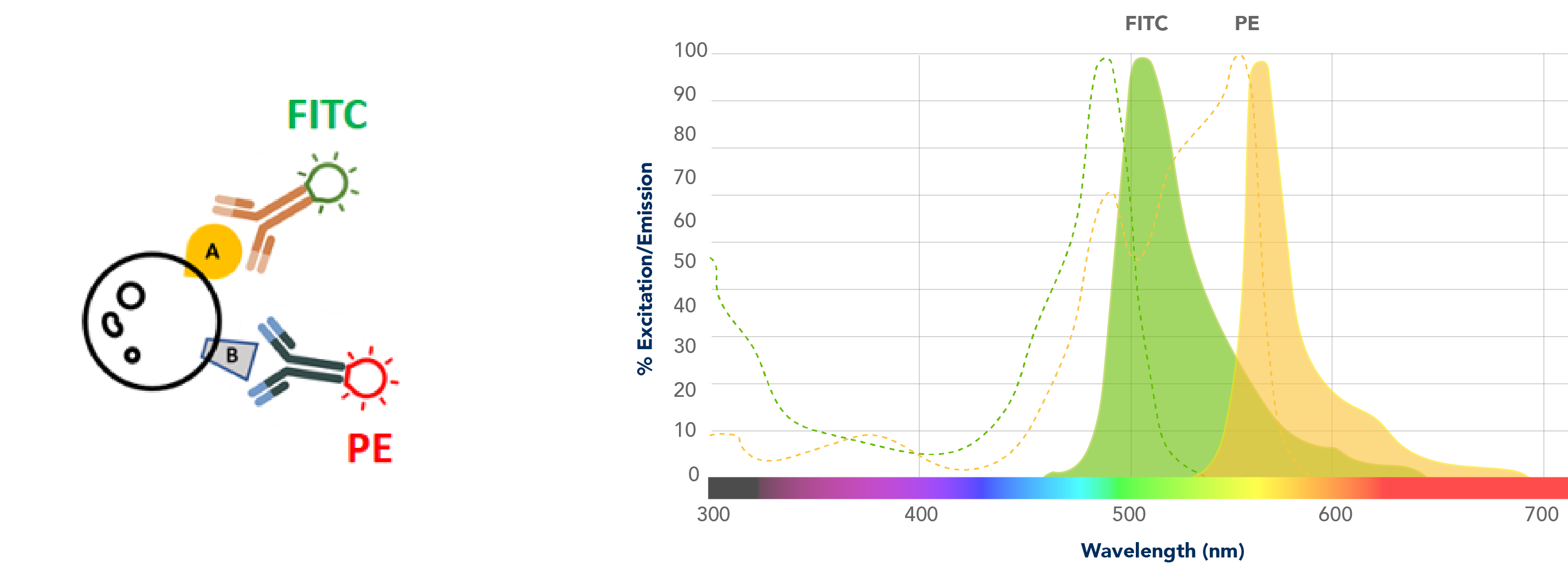
Your flow cytometer is equipped with really good filters. Any of this spectral overlap – and there’s always going to be some overlap – these filters will get rid of a lot of these nonspecific overlapping signals. But despite that, you can appreciate that there is a good deal of overlap. This spill-over on the left, something that is seen through the FITC filter, is coming in from PE, and the spill-over on the right that is seen through the PE filter is an overlap that is coming in from FITC.

How would you know that these double positive cells are real positive cells and not just spillover from this nonspecific spectral overlap? So, this is a really big strength of flow cytometry: you can correct the spectral overlap using single color controls. In this little example, your single-color controls are going to be unstained cells or something unstained to give you a negative for the background, and controls that are stained with only one antibody at a time. For example, cells that are stained with just the FITC-positive antibody and just the PE-positive antibody.
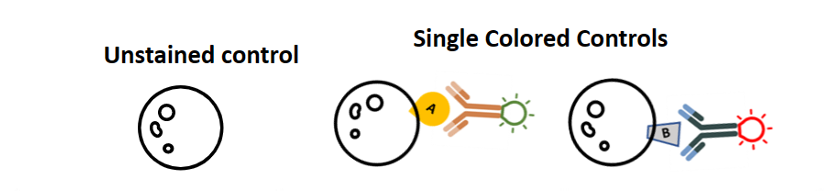
One really common mistake that users make is that they make single-color controls, but they fail to include an unstained control. You always need to have an unstained to define a negative. You cannot use the peak from your single-color controls as your negative control.
Compensation
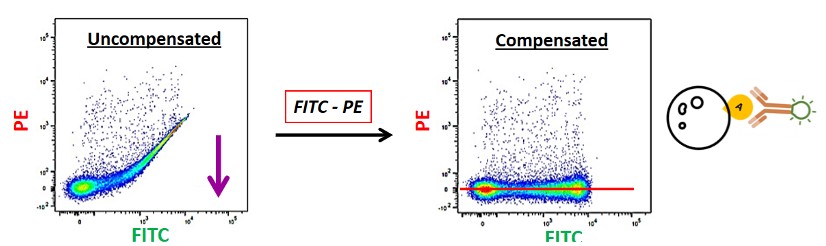
Now suppose that I run my FITC-labeled single-color control. I know I have only labeled in FITC, so I should not see anything in PE. But when I run my sample, this is what my data looks like. I have this FITC-positive, PE-positive, which I know is not real. This is because some FITC is going into PE. So, I have to remove this FITC spillover from my PE channel, and I can do that with compensation and bring this PE-positive signal back to zero. So now, everything that is fluorescing is in FITC.
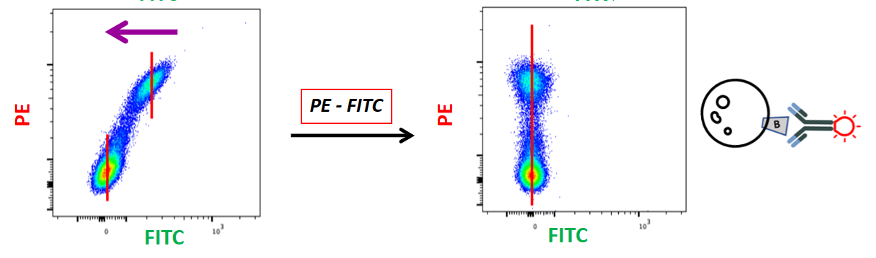
Similarly, if I run my PE-positive control and I see a FITC-positive signal, I know this is not real. This is signal coming from PE into FITC. So, I will then subtract this PE fluorescence from the FITC channel and get everything in the PE channel with nothing in the FITC channel.
This is what is known as compensation controls. And what we need to understand is that “PE minus FITC” and “FITC minus PE” are two different things. You are looking at two different lasers and two different spillovers in this way.
Tips
What should you use to stain for single-color controls? My rule of thumb is that a good single-color control should have a distinct peak for a negative and a distinct peak for a positive, so any decision that you make is going to be based on that framework.
A lot of people like using cells. The advantage is obviously that if you’re using the same cells as your actual experiment, your forward scatter and autofluorescence properties will be the same, so you can look at what they look like and how much they can contribute to your actual samples. The disadvantage is that this is obviously wasteful. You may not always have enough cells to designate into your actual test samples and separate single-color controls. And one of the major disadvantages is that biological data does not have clean peaks. You always have a smear. It’s very hard to get clean positive and negative controls or peaks from cells, and it’s very important that you have clean compensation controls.
A way to get over this hurdle is by using beads. I really, really like using beads. This makes for consistent staining, you can use your cells only to stain what you want to look for, and beads are really well separated. This is an example of cells versus beads stained with the exact same antibody, and you can see that the cells have these little smears where you don’t know where your negative ends and where your positive starts, but the beads have a clear positive and negative peak. And so, beads are always my go-to choice for compensation. The disadvantage is obviously that your autofluorescence parameters and your forward scatter and side scatter parameters will be different.

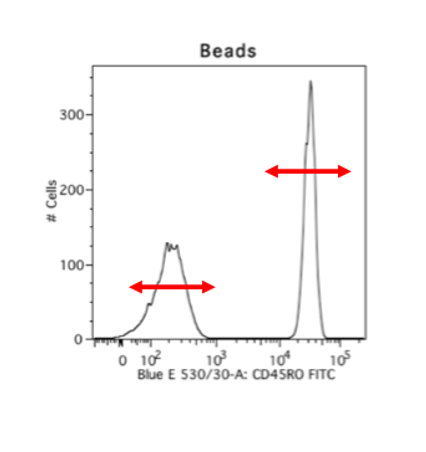
When you set up your compensation settings, this is also when you choose how bright your lasers are going to be, so where your positive peaks lie, and the stronger the laser you use, the more spectral overlap. Your compensation settings are going to be tied with how you base the voltages of your actual stained cells, like your PE or FITC. Once you come back and apply your compensation settings, if you use beads, you can still change the forward and side scatter parameters to fit your cells, because your forward and side scatter voltages don’t go into compensation. It’s just its size. But do not change any of the parameters that you have set or any of the actual lasers that are fluorescing for your targets of interest.
Dr. Puntambekar was previously an Assistant Research Professor and Scientific Manager of Flow Cytometry Core Services at Stark Neurosciences Research Institute at the Indiana University School of Medicine.
Related Content
Ask a Flow Cytometry expert: your questions answers

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.