Mimicking the Extracellular Matrix Through Electrospinning
Written by Elizabeth Byers, PhD Candidate at The Pennsylvania State University
What is Electrospinning?
Electrospinning is a highly accessible, cost-effective, and flexible technique that can easily create mats containing fibers with a large range of diameters. The process utilizes an electrostatic field in order to move fluids.
What makes electrospinning such an attractive technique in the life sciences is its ability to create nanofibers with micron and nanoscale diameters which recapitulates fiber sizes found within the extracellular matrix (ECM). Surfaces made using electrospinning are used in a variety of biomedical applications such as drug delivery, tissue engineering, wound healing, and purification5. It is also useful as a tool in basic cell biology to investigate cell-ECM interactions include cell adhesion, migration, proliferation, and differentiation in normal and pathological states6,7.
Components of Electrospinning
Electrospinning is a rather simple technique that nearly anyone can perform – all you need are a polymer solution, syringe pump, syringe, needle, voltage source, and metal collecting surface (Fig. 1). Given so few components are needed, the technique can be adopted by any lab for a very low start-up cost, and the maintenance of the components is minimal. The most important part of electrospinning is grounding your voltage source to make the process safe, as voltages in the kV range are used.
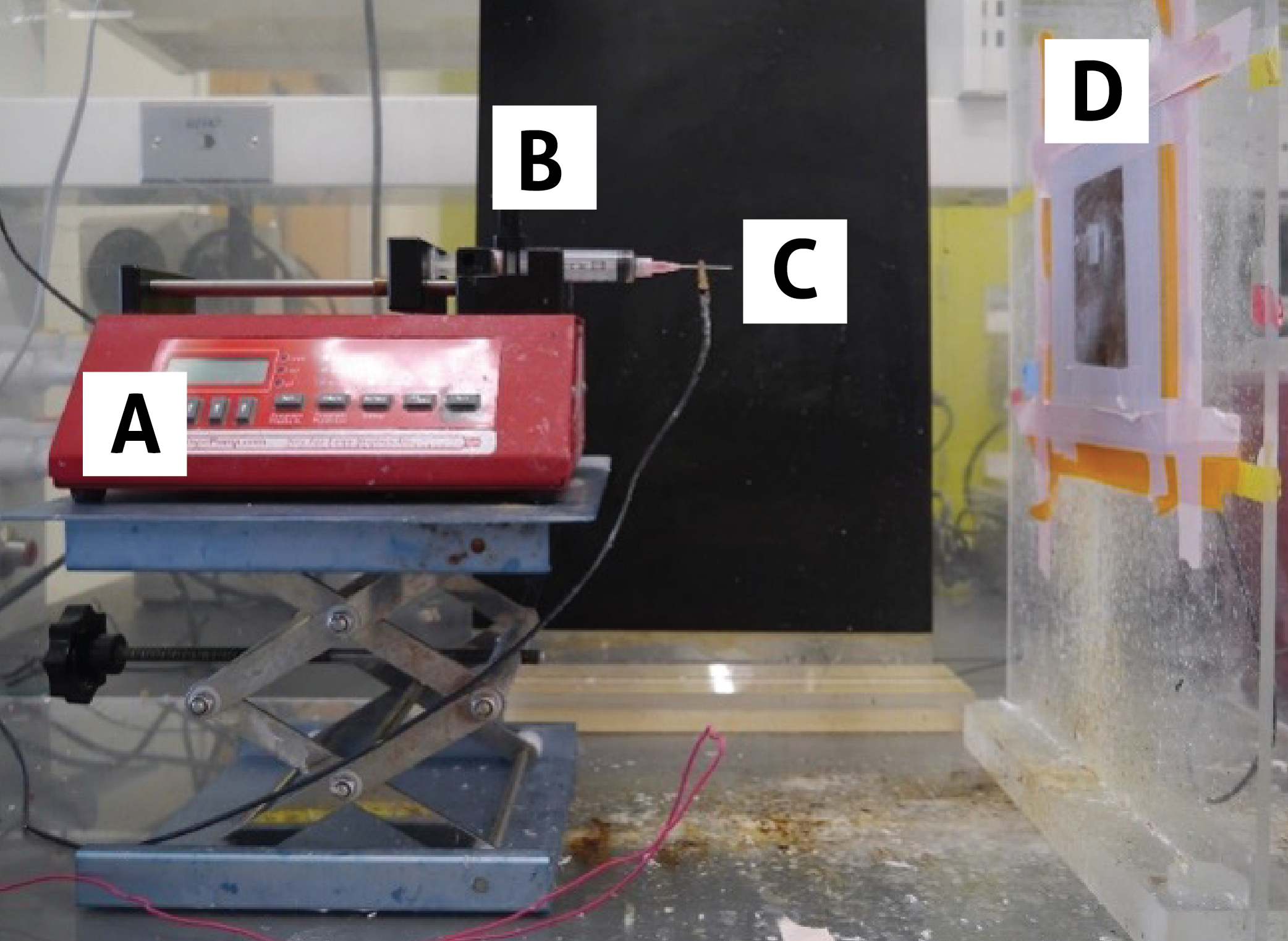
Figure 1. Example electrospinning setup. Shown are A) syringe pump, B) syringe containing a polymer solution, C) needle attached to voltage source via alligator clip, and D) flat copper collection plate Source: Elizabeth Byers, Brown Lab, Penn State University
How does it work?
During electrospinning, a polymer solution is first pumped through a syringe into the attached needle until a small droplet appears at the needle tip. This droplet must form in order for fibers to be drawn out of the polymer solution towards the collector. When a voltage is applied to this droplet, a Taylor cone is formed, and fibers are pulled from the polymer solution (Fig. 2A)3. The cone resembles a tornado with the tip of the funnel cloud emanating from the needle. Within the Taylor cone, fibers cross the distance from needle to collector, but due to bending instability in the fiber and the electrostatic repulsive forces generated by the fiber itself, the fiber whips and bends, creating a randomly oriented mat of fibers (Fig. 2B).
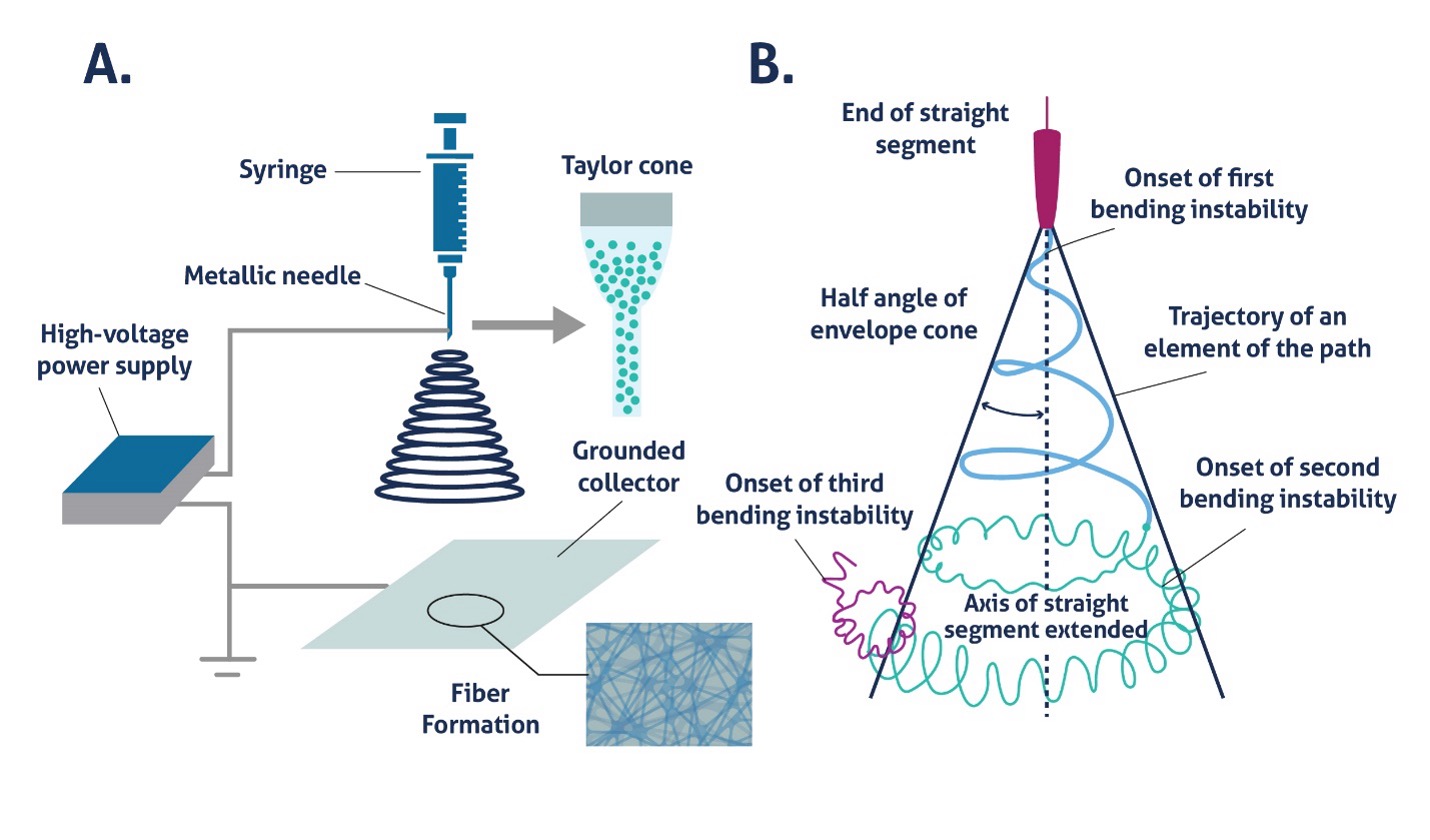
Figure 2. Electrospinning apparatus and Taylor cone (A) Illustration of electrospinning setup similar to that shown in Figure 1 (B) Taylor cone diagram showing the effect of bending instability on fiber trajectory4
Each component of the electrospinning setup is modular and can be changed to affect fiber morphology. Flat collectors, such as plates, can be used to make randomly oriented fibers, while drums or other rotating collectors can be used for producing aligned fibers (Fig. 3A). Changing physical parameters such as the distance from needle tip to collector, needle gauge, and pump speed can alter the size of fibers, as can environmental parameters such as ambient humidity, temperature, and airflow. Finally, the type of polymer, its molecular weight and solubility, solvent vapor pressure, and solution concentration, conductivity, and surface tension can control fiber size (Fig. 3B)2.
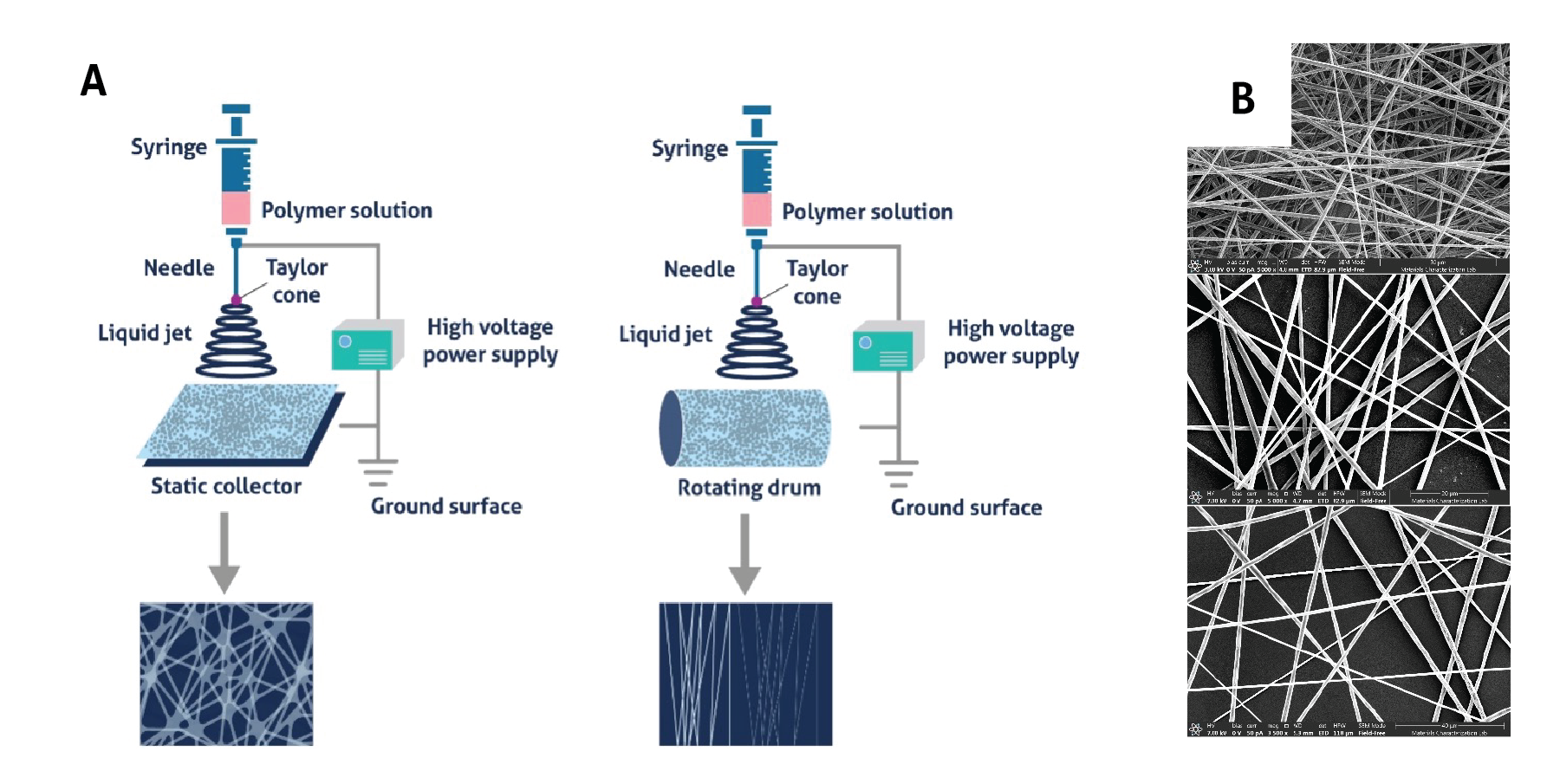
Figure 3. Impact of parameter selection on nanofiber morphology (A) Static collectors are used to create randomly oriented nanofibers while rotating collectors allow nanofibers to align, Source: Unknown (B) Increasing polymer concentration increases the diameter of the spun fibers, Source: Elizabeth Byers, Brown Lab, Penn State University
Applications
Drug Delivery
Nanofibers make attractive drug delivery systems due to high surface area, which makes it possible to load large numbers of molecules or combine multiple types, and the ability to be functionalized in a myriad of ways. Given that the applications for drug delivery are highly varied, so are the uses of nanofibers. Examples of molecules combined with nanofibers include antimicrobial8 and antifungal9 drugs, anticancer10 drugs, growth factors11, and nucleic acids12.
Tissue Engineering and Wound Healing
Tissue regeneration is likely the most popular application for nanofibers and is where extensive research has been conducted. Nanofibers act as a scaffold for cells to attach and grow and can be used to both tailor cell responses to encourage differentiation, cell secretion, migration, etc. patterns of the researcher’s choosing and support bulk tissue formation, which is difficult to achieve on cell culture plastic. In particular, the ability to create aligned fibers using electrospinning has been used to create improved scaffolds for cardiac, skeletal muscle, tendon, ligament, and neural tissues, which are naturally aligned and benefit from a surface that mimics the in vivo environment. However, electrospun scaffolds are by no means limited to these applications and are also studied in the contexts of bone, skin, blood vessels, and cartilage. In addition to pure nanofiber scaffolds, electrospinning can also be combined with other techniques to create hybrid materials such as hydrogel-nanofiber13, microsphere-nanofiber14, and even metallic nanofiber15 constructs.
Cell and Cancer Biology
Nanofibers can also be used to model various aspects of the tumor microenvironment – aligned fibers can mimic the tracks formed by invasive cancer cells as they exit the tumor site, fibers with various stiffness can recapitulate changes in ECM material properties during tumor development, and fibrous mats with different pore sizes and fiber densities can be used to observe how cancer cells (as well as other cell types) migrate16.
Drawbacks
While electrospinning has many strengths, it does not come without issues. The most prominent difficulty when working with electrospinning micro- and nanofibers is achieving consistent fibers during every spinning session. Even when it may appear that all parameters are held constant, fiber morphology is affected by so many different variables, and small changes in the environment, polymer solution, or physical setup can have large consequences. To improve reproducibility, many companies now offer out-of-the-box electrospinning setups with climate-controlled spinning chambers and more automated controls, but these setups tend to run in the thousands of dollars, eliminating the cost advantage the technique offers. However, it should be noted that researchers have published free electrospinning designs intended to improve the reproducibility of the technique, while keeping costs low17–19. Another major issue is scale-up, but similarly to the issue of reproducibility, companies such as Elmarco and NanoScience Instruments (a by no means exhaustive list) have developed the Nanospider™ Lab and Fluidnatek HT, respectively, to enable high-throughput, industrial scale electrospinning.
References
- Ghosal, K., Agatemor, C., Tucker, N., Kny, E. & Thomas, S. Electrical Spinning to Electrospinning: a Brief History. RSC Soft Matter 2018-January, 1–23 (2018).
- Zhao, P. et al. Electrospun Nanofibers for Periodontal Treatment: A Recent Progress. Int J Nanomedicine 17, 4137–4162 (2022).
- Electrically driven jets. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences 313, 453–475 (1969).
- Wang, C. et al. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, Vol. 24, Page 834 24, 834 (2019).
- Rasouli, R., Barhoum, A., Bechelany, M. & Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol Biosci 19, 1800256 (2019).
- Amores de Sousa, M. C. et al. Functionalization of Electrospun Nanofibers and Fiber Alignment Enhance Neural Stem Cell Proliferation and Neuronal Differentiation. Front Bioeng Biotechnol 8, (2020).
- Liu, G. F., Zhang, D. & Feng, C. L. Control of three-dimensional cell adhesion by the chirality of nanofibers in hydrogels. Angewandte Chemie - International Edition 53, (2014).
- Sousa, M. G. C., Rezende, T. M. B. & Franco, O. L. Nanofibers as drug-delivery systems for antimicrobial peptides. Drug Discov Today 26, 2064–2074 (2021).
- Veras, F. F., Roggia, I., Pranke, P., Pereira, C. N. & Brandelli, A. Inhibition of filamentous fungi by ketoconazole-functionalized electrospun nanofibers. European Journal of Pharmaceutical Sciences 84, 70–76 (2016).
- Liu, D. et al. Necrosis of cervical carcinoma by dichloroacetate released from electrospun polylactide mats. Biomaterials 33, 4362–4369 (2012).
- Sahoo, S., Ang, L. T., Goh, J. C. H. & Toh, S. L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A 93, 1539–1550 (2010).
- Rujitanaroj, P. on, Wang, Y. C., Wang, J. & Chew, S. Y. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials 32, 5915–5923 (2011).
- Zhang, M. et al. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J Mater Sci Technol 162, 157–178 (2023).
- Ionescu, L. C., Lee, G. C., Sennett, B. J., Burdick, J. A. & Mauck, R. L. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials 31, 4113–4120 (2010).
- Sang, W., Zhang, R., Shi, X. & Dai, Y. Advanced Metallized Nanofibers for Biomedical Applications. Advanced Science 10, 2302044 (2023).
- Huang, W. Y., Suye, S. I. & Fujita, S. Cell Trapping via Migratory Inhibition within Density-Tuned Electrospun Nanofibers. ACS Appl Bio Mater 4, 7456–7466 (2021).
- Waqas, M. et al. Design and development of a nozzle-free electrospinning device for the high-throughput production of biomaterial nanofibers. Med Eng Phys 92, 80–87 (2021).
- Viet, T. N., Thai, D. M., Phuc, P. T. & Van Toi, V. Design and Fabrication of a Complete Electrospinning System. IFMBE Proc 85, 85–99 (2022).
- Ryu, H. Il et al. Uniform-thickness electrospun nanofiber mat production system based on real-time thickness measurement. Scientific Reports 2020 10:1 10, 1–10 (2020).
Related Content
How to culture mouse primary cerebral microvascular endothelial cells
Tips and tricks for iPSC culture
Overview of the vascular system and the importance of endothelial cells
What are recombinant antibodies?
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

