A Guide to Studying Wild-Type and Mutant Proteins
Written by Suparba Roy, PhD Candidate at Université Laval
Introduction
Proteins are fundamental building blocks of life. Here, we discuss how researchers study the functional and structural aspects of wild-type and mutant proteins to understand the human body in healthy and diseased states.
Although science fiction movies portray mutations leading to “superhuman” physical characteristics, real-life mutations cause less exciting changes. For instance, mutant proteins may suppress, inhibit, or overexpress wild-type proteins or may gain new functions that are completely different from those of wild-type proteins. Mutations are changes in a nucleotide sequence caused by deletion, substitution, or insertion of a nucleotide, resulting in a change in nucleotide bases. This may alter an amino acid sequence that could lead to conformational/structural and regulatory changes related to the functions of that protein. For example, if an enzyme (all enzymes are proteinaceous in nature, except for ribozymes!) undergoes a structural mutation, the active/catalytic site may no longer be functional as substrates would fail to bind to the active site.
Although researchers have put tremendous efforts into learning about the structural and functional aspects of proteins, quite a bit remains unexplored and has the potential for discovery.
How to study protein functions?
Protein functions are categorized under two broad classifications:
- Functional analysis and Structural analysis.
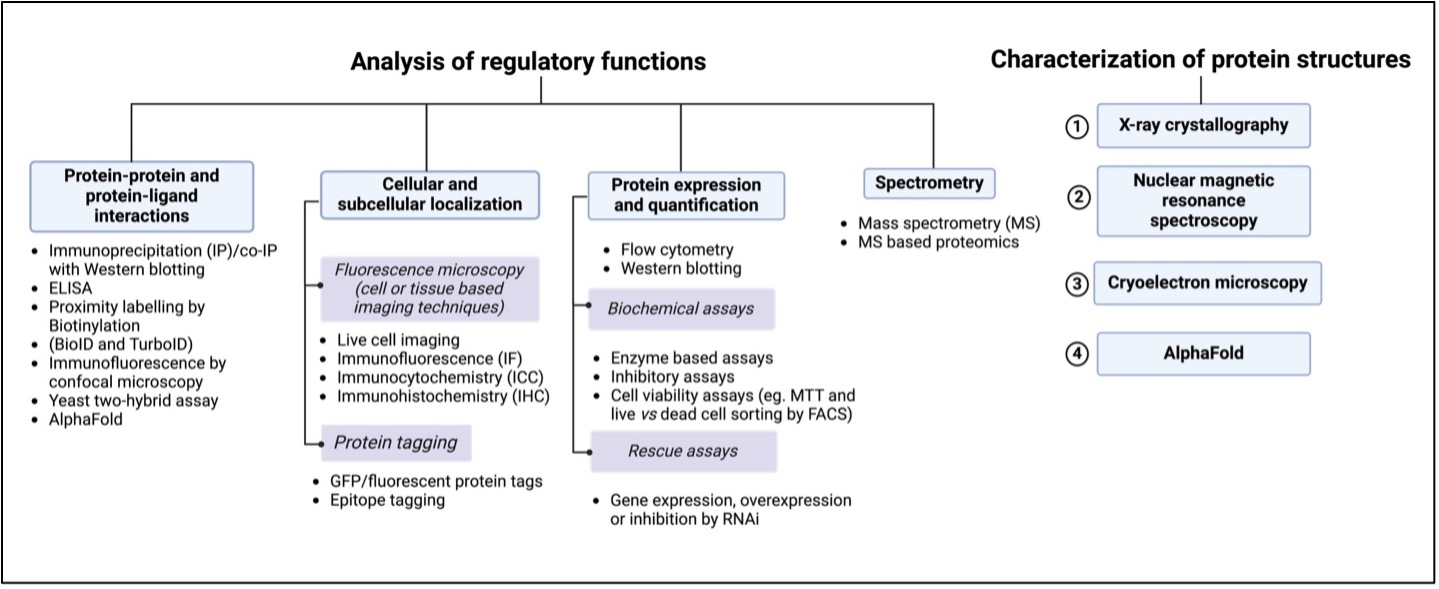
Figure 1. Guide to studying protein functions and structures.
Functional assays: The regulatory functions of proteins can be studied by functional assays to help understand protein activity under different conditions. To study proteins from biological samples, proteins can be extracted and purified. Protein purification is broadly obtained by first extracting from cells, centrifugation, and chromatography or SDS-PAGE, and finally, the quantification of the purified protein. Interestingly, in contrast to cell lysate preparation, which is typically required for western blotting, in-cell western is an immunocytochemical tool that does not require lysate preparation and detects proteins endogenously. Furthermore, endogenous proteins can also be studied by fixed cell or fixed tissue imaging. In the following sections, we dive deeper into various techniques that are used for studying proteins in wild-type and mutant samples, with or without the presence of a drug or other molecules.
To effectively study wild-type and mutant proteins, genetically engineering the genome is often a common practice in labs. For example, transfection (non-viral system) and transduction (viral vector-based system) introduce mutations by inserting nucleic acids in the host genome. These are further validated by different protein expression validation tools. These techniques allow us to study native and mutant protein functions.
1. Binding assays help us in studying protein interactions with other nucleic acids like DNA and RNA, ligands, and other proteins. Hence, mutants and wild-types can be studied by performing a comparative study of these protein interactions. Some of the most commonly practiced binding assays are immunoprecipitation (IP) and co-IP, mass spectrometry, biotinylation of protein(s) of interest, enzyme-linked immunosorbent assay (ELISA), western blotting, in-cell western, and yeast two-hybrid assays. Therefore, binding assays are widely used to understand the effects of mutations on protein functions. For example, binding assays provide valuable information when wild-type proteins exhibit their canonical functions, whereas mutant proteins fail to express these functions by not interacting with their respective ligands, substrates, or other binding partners. Furthermore, binding assays allow researchers to explore broader aspects of disease pathophysiology by studying disease-causing mutations in proteins that alter their function. These assays are extensively used to study the effect of drugs on protein functions that not only underscore the molecular mechanism and functions of wild-type and mutant protein expression but may also help identify potential drug candidates for treating diseases.
2. Studies have shown that the functions of proteins are dependent on their spatiotemporal localization. This phenomenon can be due to different signaling cascades, post-translational modifications, stress-induced interactions or activities, and so on, and hence an important aspect to learn about their functions with respect to their localization. Subcellular and intracellular localization can be studied by microscopy. Although it is a time-consuming and labor-intensive approach, it is still one of the most sought after and effective approaches and is widely acknowledged due to its dependability. Some common examples are immunofluorescence (IF)-based microscopy techniques like immunocytochemistry (ICC) and immunohistochemistry (IHC). In these experiments, proteins of interest can be visualized in cells or tissues using primary and fluorescently labeled secondary antibodies. With the use of microscopy-based techniques, wild-type proteins can be differentiated from mutants by studying their expression, localization pattern, interaction with other proteins in the vicinity of proteins of interest, and cell/tissue morphology. Moreover, mutations can sometimes lead to “gain-of-function” like phenotypes that are different from native or canonical wild-type functions. Whether a protein exhibits “loss-of-function” or “gain-of-function” like phenotype due to a mutation can also be studied by microscopy. Additionally, if mutants influence cell proliferation or cell death, cell polarity, colony formation, and so on, can be studied by comparing with wild-type proteins using microscopy-based techniques.
3. In order to study endogenous protein expression or molecules of interest, fluorescence-activated cell sorting (FACS) is an advance flow cytometry technique that allows the separation of populations of cells from a heterogeneous mixture of cells based on the simple phenomenon of light scattering characteristics of each cell that bears a specific fluorophore. Researchers use highly specific fluorophore-tagged antibodies, which allow them to analyze each data point, one cell at a time emitting light at a specific wavelength of fluorescence emission. Taking advantage of this feature, researchers study the difference between population of cells with wild-type and mutant protein expression. For example, wild-type and mutant proteins are studied by FACS to investigate cellular aspects like oxidative stress and mitochondrial membrane potential that offer a wealth of information on cellular health. Certain mutations induce stress, eventually leading to cell cycle arrest, cell death, cell senescence or quiescence, and some mutations exacerbate DNA damage repair mechanisms, perturb and bypass necessary cell cycle checkpoints, and promote cell proliferation, leading to tumorigenicity. Such conditions are well identified by FACS, which sheds light on how wild-type and mutant proteins affect overall cell biology and determine cell fate.
4. A cell’s fate is based on several factors. Studying wild-type and mutant protein expression for cell viability allows us to learn about cellular health and gain a deeper understanding of cellular and molecular activities. Mutations can alter a protein’s native functions and affect cell viability. Some common assays for studying cell viability are MTT assay for measuring metabolic activity, trypan blue dye and propidium iodide staining (by FACS) for live/dead cell staining, and ATP/luminescence-based assays for viable cells (as live or healthy cells usually produce more ATP!). These techniques not only offer a robust approach to study the difference between wild-type and mutant protein functions but also how it affects overall cellular health.
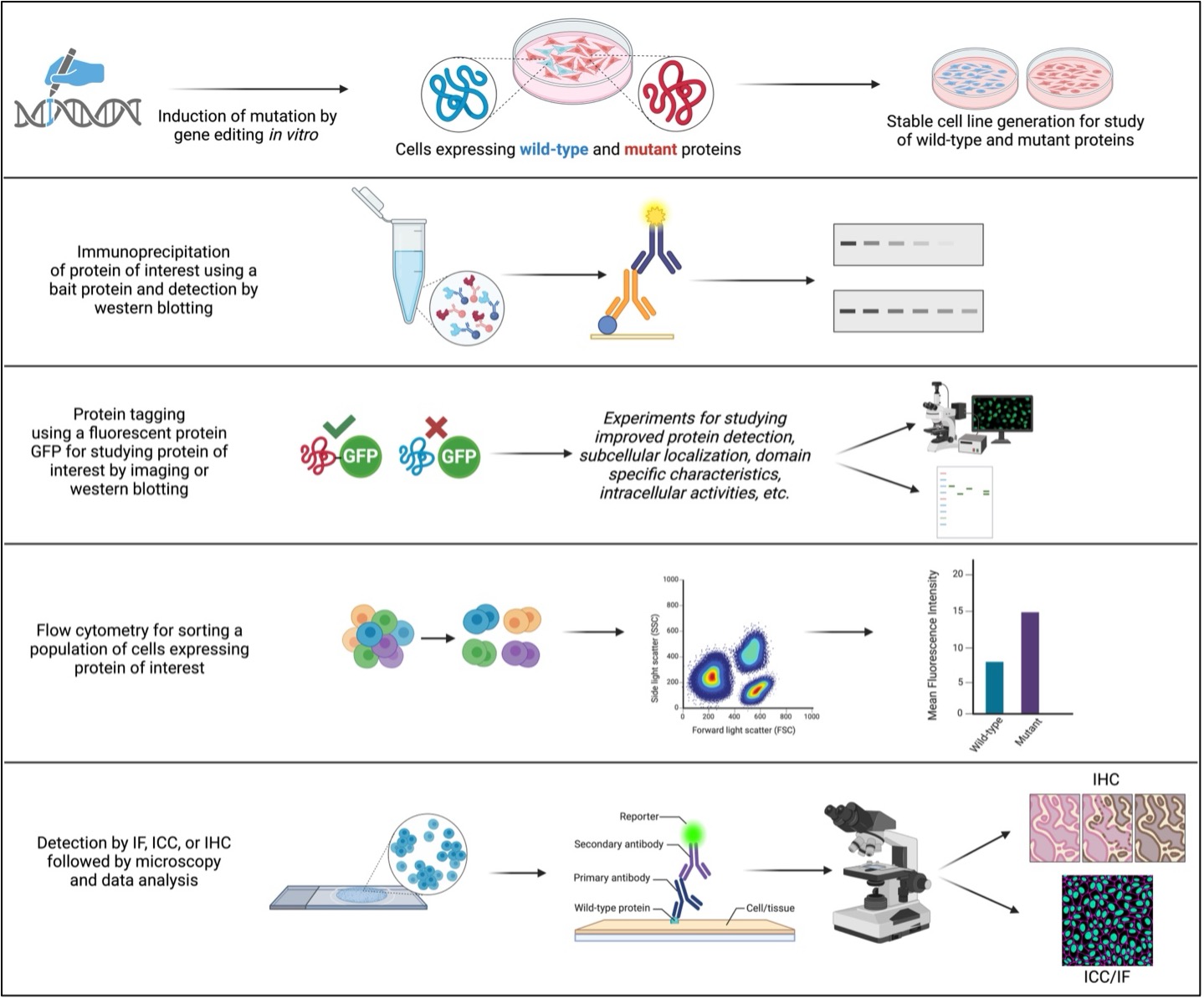
Figure 2. A schematic of common experiments for studying wild-type and mutant proteins and key steps involved.
(Note: all illustrations are representative of generic steps in the mentioned experiments and need necessary modifications based on the study, samples, and/or objectives.)
Structural analysis is the characterization of 3-dimensional protein structure based on the protein folding mechanism-a vital step in post-translational modification. Noteworthily, protein misfolding is a well-known contributor of a wide array of mutations as it affects protein stabilization and is a hallmark of cancer. Studying protein conformational changes is, therefore, a crucial aspect of exploring protein functions. Wild-type and mutant conformers can both exhibit different sets of functions, which could be due to mutations that render inactive mutant proteins, failure in binding activity to other molecules, or gain-of-function like activity. Over the years, analyses of protein structures and conformers have been achieved experimentally by X-ray crystallography, nuclear magnetic resonance spectroscopy, and cryoelectron microscopy. Although these methods have very high accuracy, they are time-consuming and expensive. More recently, artificial intelligence (AI) with biology has addressed these two problems. Google DeepMind designed an AI-based computational biology tool called AlphaFold that can predict 3-D protein structures in minutes with an accuracy of up to 80%. Its applications have helped estimate the effects that genetic mutations have on a protein’s structure and function, discover drugs that bind to protein pockets, model interactions of proteins that engage in protein-protein interactions, and engineer proteins with new functions for drug targets, biotechnology, agriculture, and the broader environment. AlphaFold represents a significant breakthrough in the analysis of protein structures.
Conclusion
Understanding the functional consequences of proteins and their mutations is crucial for comprehending the molecular basis of various mechanisms of different biological systems. This guide has explored a range of experimental techniques, from biochemical assays like immunoprecipitation and western blotting to sophisticated microscopic and flow cytometric approaches, which are widely practiced. By carefully selecting and applying these methods, researchers can investigate how mutations impact protein expression, localization, interactions, and, ultimately, cellular function. The study of wild-type and mutant proteins is an ongoing and dynamic field, not only in terms of drug discovery and understanding diseases but also in knowing the unknown biology and natural science. Various advancements in technologies such as CRISPR-Cas9 gene editing, deep mutational scanning, and high-throughput screening are continually expanding our ability to investigate protein function and identify novel therapeutic targets. Future advancements will continue to refine our ability to study protein functions and the impact of mutations.
References
- Mishra, M., Tiwari, S., Gomes, A. Protein purification and analysis: next generation Western blotting techniques. Expert Rev Proteomics. 2017 Oct;14(11):1037-1053. Doi: 10.1080/14789450.2017.1388167. Epub 2017 Oct 13. PMID: 28974114; PMCID: PMC6810642.
- Deng, C., Xiong, X. & Krutchinsky, A. Unifying Fluorescence Microscopy and Mass Spectrometry for Studying Protein Complexes in Cells. Mol Cell Proteomics. 2009 Jun;8(6):1413-23. Doi: 10.1074/mcp.M800397-MCP200. Epub 2009 Mar 5. PMID: 19269952; PMCID: PMC2690482.
- Krohn, R. The colorimetric detection and quantitation of total protein. Curr Protoc Cell Biol. 2011 Sep;Appendix 3:3H. doi: 10.1002/0471143030.cba03hs52. PMID: 21898335.
- Picot, J, Guerin, C.L., Le Van Kim, C. & Boulanger, C. Flow cytometry: retrospective, fundamentals and recent instrumentation. Cytotechnology. 2012 Mar;64(2):109-30. Doi: 10.1007/s10616-011-9415-0. Epub 2012 Jan 21. PMID: 22271369; PMCID: PMC3279584.
- Dobson, C.M. Protein folding and misfolding. Nature426, 884–890 (2003). https://doi.org/10.1038/nature02261
- Xiong, C., Ling, H., Hao, Q. & Zhou, X. Cuproptosis: p53-regulated metabolic cell death?. Cell Death & Differ 30, 876–884 (2023). https://doi.org/10.1038/s41418-023-01125-0
- Gerasimavicius, L., Livesey, B.J. & Marsh, J.A. Loss-of-function, gain-of-function and dominant-negative mutations have profoundly different effects on protein structure. Nat Commun 13, 3895 (2022). https://doi.org/10.1038/s41467-022-31686-6
- Wade, M., Méndez, J., Coussens, N.P., Arkin, M.R. & Glicksman, M.A. Inhibition of Protein-Protein Interactions: Cell-Based Assays. 2017 Nov 20. In: Markossian, S., Grossman, A., Arkin, M., et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK464632/
- Jumper, J., Evans, R., Pritzel, A., et al.Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). https://doi.org/10.1038/s41586-021-03819-2.
- Alberts, B. et al. Molecular Biology of the Cell (7th Edition), Garland Science, 2022.
Related Content
Want to upgrade your immunofluorescence workflow? Go Direct!
Intracellular Flow Cytometry Staining Protocol
A Guide to Building a Direct Sandwich ELISA
Tips for detecting phosphoproteins by western blot
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

