Cytokines and Growth Factors
Human cell (HEK293) expressed cytokines and growth factors with high bioactivity, stability, lot-to-lot consistency, and native human conformation & post-translational modifications.
Our human expression system ensures that proteins have native conformation and post-translational modifications to optimize biological activity. No expression tags, xeno-free…just high quality proteins.
Animal component free
Endotoxin free
Xeno free
Tag free
Carrier free
HumanKine® recombinant proteins are produced in HEK293 cells using animal free components
Proteins co-expressed in bacteria will not possess post- translational modifications, e.g., phosphorylation or glycosylation. For activity, many proteins require glycosylation and processing available exclusively in eukaryotic systems; specifically human systems for authentic human proteins.
All Humankine® recombinant proteins are produced in Proteintech's in-house cGMP grade laboratory adhering to strict quality control regulations.
-
Authentic human proteins
-
Native human conformation & post-translational modifications
-
High bioactivity & stability
-
Seamless transition from research to cGMP
Research Grade vs cGMP Grade
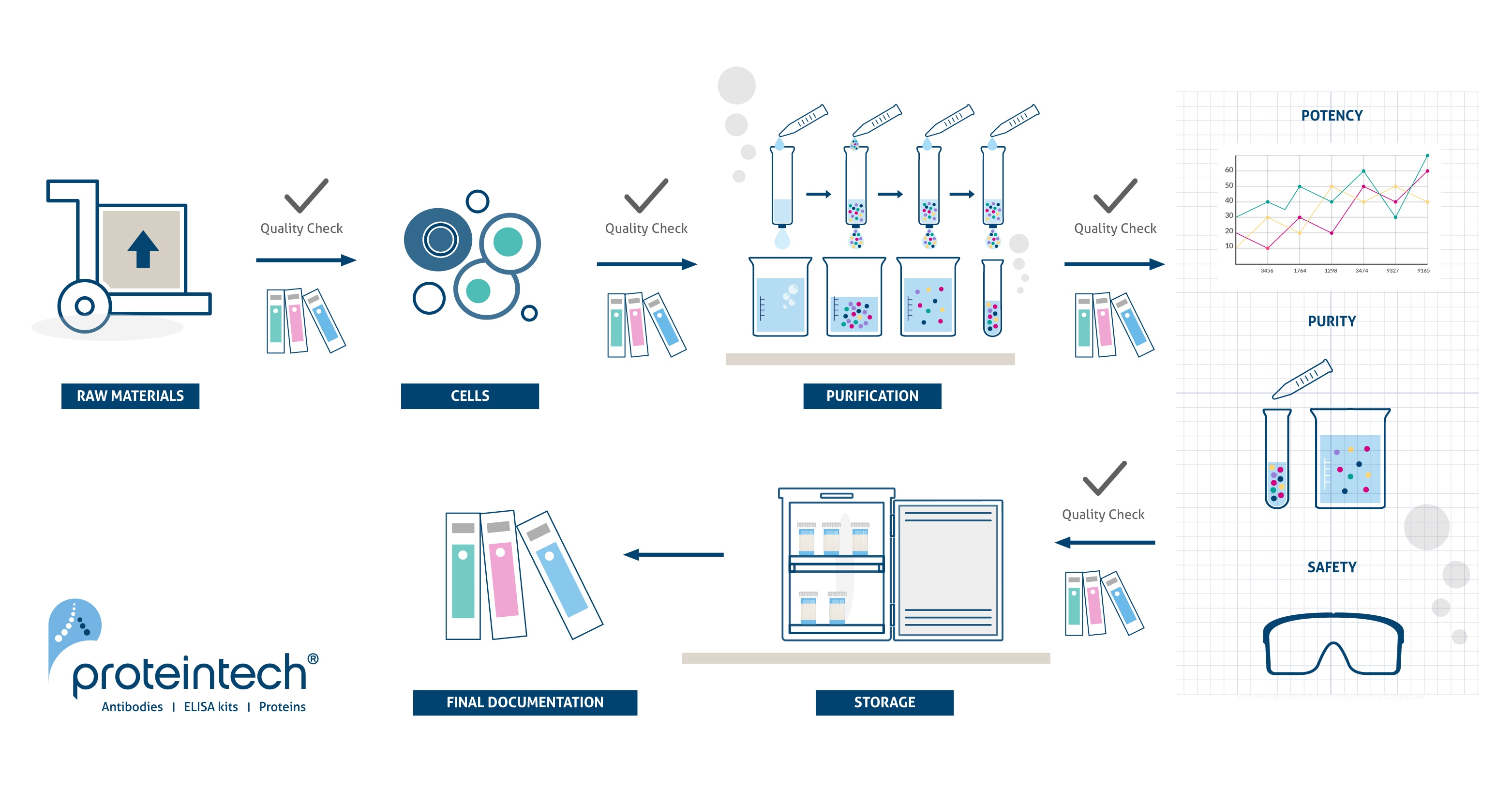
In order to facilitate a smooth transition from preclinical to clinical, Humankine® offers a wide range of Research Use Only (RUO) and cGMP grade recombinant proteins which are exclusively expressed and purified from HEK293 human cells. The RUO and cGMP products are both manufactured using the same expression systems, production process and operating procedures in a cGMP facility. This guarantees that there is no variability in the quality and performance of these products.
The key differentiator of our cGMP products from RUO is that we adhere to a rigorous quality process that is ISO13485 certified and cGMP compliant. The cGMP grade products come with extensive documentation, thorough QC testing and traceability. The RUO grade offers reliable products which are more cost-effective during early research and development.
cGMP Manufacturing Process
Frequently Asked Questions
What is the stability of HumanKine proteins?
Unless specified otherwise in the product information sheet, our products are formulated to ensure the stability of lyophilized proteins at room temperature. It is advised to store lyophilized products at -20°C to -80°C. For reconstituted solutions of most products, short-term storage at 4°C is recommended. Extended storage of the protein solution should be at -20°C to -80°C. Keep in mind that each freeze/thaw cycle may lead to some protein denaturation, so it is not recommended to subject aliquots to multiple freeze/thaw cycle.
Why are some proteins reconstituted in acidic buffer?
Acid reconstitution buffer is especially important for proteins with a high isoelectric point, making the molecule hydrophobic at neutral pH. Failure to use the recommended buffer may result in incomplete dissolution of the cytokine or its potential loss on the surfaces of labware such as pipet tips, vials, and test tubes. Diluting the protein aliquots to the working concentration in the appropriate buffer for a specific application helps neutralize the small amount of acid added, ensuring the safe use of the protein with live cells.
Is it ok to replace Human Serum Albumin (HSA) with Bovine Serum Albumin (BSA) in reconstitution buffer?
Bovine serum albumin (BSA) can be used instead of Human Serum Albumin (HSA) in reconstitution buffer. We recommend to use recombinant HSA to keep the product animal component free. If your experiment will not be impacted by the presence of animal derived components, BSA containing buffer can be used for reconstitution.
Can HumanKine cytokines cross-react with mouse cells?
Except for a few cases, the majority of human cytokines exhibit activity on mouse cells. While many mouse cytokines can act on human cells, their specific activity might be lower compared to the corresponding human cytokine. Species specificity is observed in certain cytokines, such as interferons, GM-CSF, IL-3, and IL-4, which have minimal to no activity on non-homologous cells. In contrast, FGFs and neurotrophins are highly conserved, displaying robust activity on cells from various animal species.
How is HumanKine protein bioactivity calculated in international units(IU)?
International Unit (IU) values for our Humankine products are obtained by conducting multiple side-by-side comparisons with the WHO Reference Standard within our biological activity assay. This approach involves performing several comparison tests to account for potential outliers arising from variations in the assay, such as differences in the product, handling, assay protocol, and more. To learn more about international unit calculation, read our blog.
Why does my lyophilised protein vial appear to be empty?
The lyophilized product may have shifted from the vial's bottom during shipping/handling, scattering on the vial wall or cap. A firm tap on the bench or a brief spin in a centrifuge can help reposition the lyophilized product back to the vial's bottom. Certain pellets may present themselves as a minimal amount of material or a transparent film, attributed to the characteristics of the original buffer formulation. This appearance is typical for numerous proteins and is considered normal.
Resources
Mesenchymal Stem Cell
Wnt Signaling
Induced Pluripotent Stem Cell
TGF-beta Signaling
Haematopoietic Stem Cell
IL-6
BMP-2
Independent research demonstrates HEK293 derived BMP-2 is more active and stable than either E.coli or CHO derived BMP-2.
IL-2
Proteintech's HumanKine recombinant IL-2 is 40x more active than leading competitor product.
Read More
Wnt3A
High purity, high activity Wnt3A, without the high price.
Read More
Product Flyer
HumanKine Cytokines and Growth Factors.
Read More
Product Flyer
cGMP Grade HumanKine Cytokines and Growth Factors.
Read More
Articles
ISO 13485 certification - cGMP compliant proteins
Proteintech announces ISO 13485 Certification for its HumanKine® Human Cell-expressed Cytokines and Growth Factors.
Top 4 considerations when choosing your recombinant proteins
Due to the importance in cell culture and therapeutic systems, there are several considerations to evaluate when choosing a recombinant protein for your own cultures.
What do you get when you buy a cGMP-Grade product?
cGMP manufacturing, benefits, and ISO certifications explained.
6 reasons why HEK293 proteins are superior for clinical applications
Find out the benefits of HumanKine® recombinant proteins, cytokines and growth factors.
ISO 13485 certification - cGMP compliant proteins
Proteintech announces ISO 13485 Certification for its HumanKine® Human Cell-expressed Cytokines and Growth Factors.
Top 4 considerations when choosing your recombinant proteins
Due to the importance in cell culture and therapeutic systems, there are several considerations to evaluate when choosing a recombinant protein for your own cultures.
What do you get when you buy a cGMP-Grade product?
cGMP manufacturing, benefits, and ISO certifications explained.
6 reasons why HEK293 proteins are superior for clinical applications
Find out the benefits of HumanKine® recombinant proteins, cytokines and growth factors.
New cGMP Laboratory
Proteintech announces completion of its new cGMP laboratory.
FGF Basic TS
Thermostable FGF does not require media changes over the weekend.