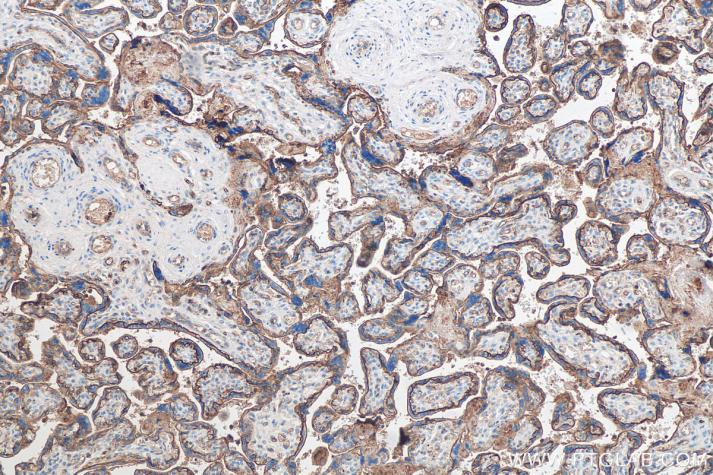
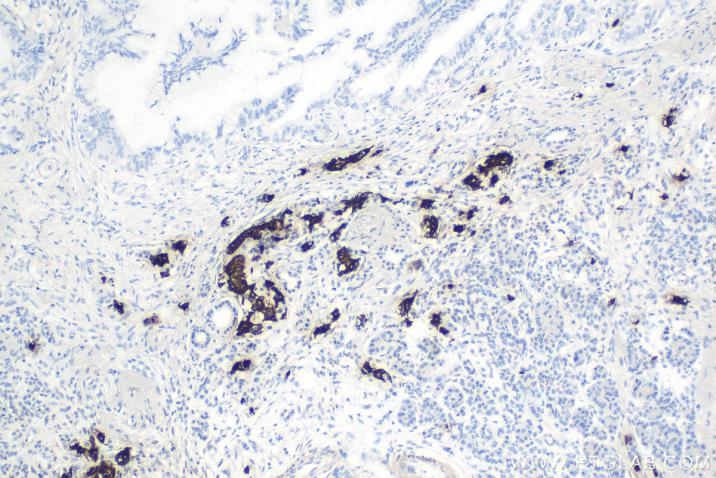
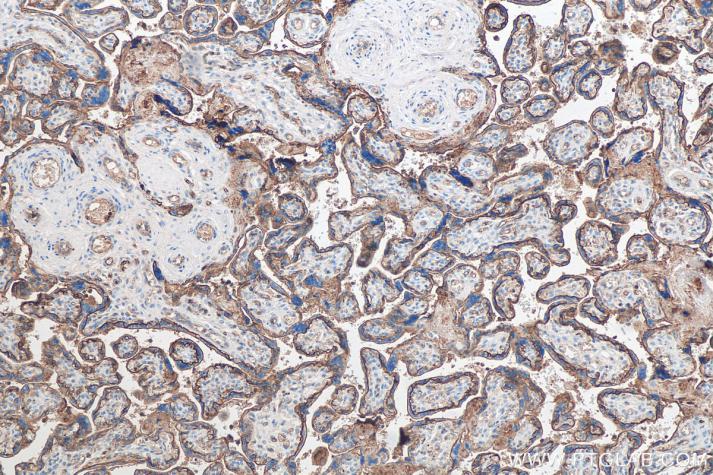
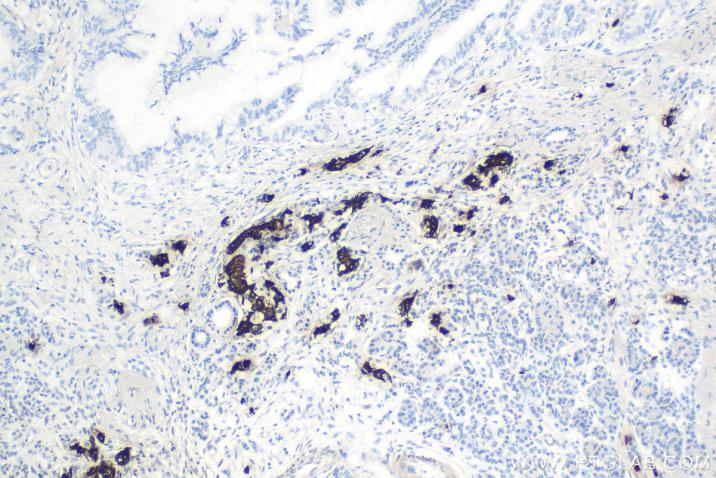
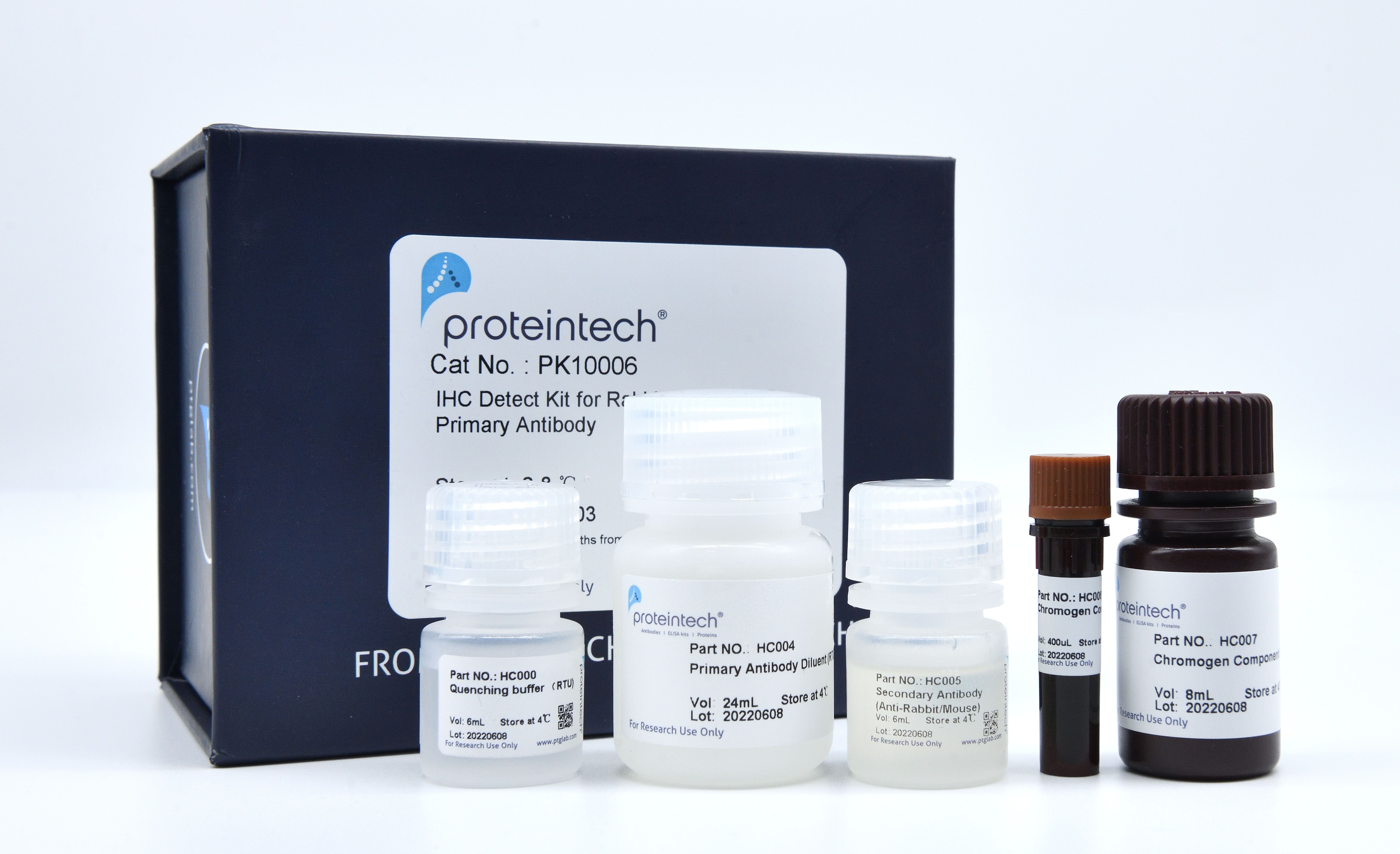
Product Information
The PK10006 kit is designed to facilitate signal detection during IHC analysis of FFPE tissue samples using primary antibodies raised in rabbits/mice. The kit consists of Polymer HRP-Goat anti- Rabbit/Mouse Secondary Antibody and an ultra-sensitive Chromogen for highly sensitive detection of rabbit/mouse primary antibodies. The kit also contains an optimized primary antibody dilution buffer that preserves primary antibody activity and helps lower non-specific background staining.
Note:
* If you are planning to use a rabbit primary antibody for staining mouse samples, we recommend using our IHC Detect Kit for Rabbit Primary Antibody (Cat.NO. PK10009).
* If antigen retrieval buffer, counter staining reagent and mounting media are needed, please see our IHC Prep & Detect Kit for Rabbit/Mouse Primary Antibody (Cat.NO. PK10019).
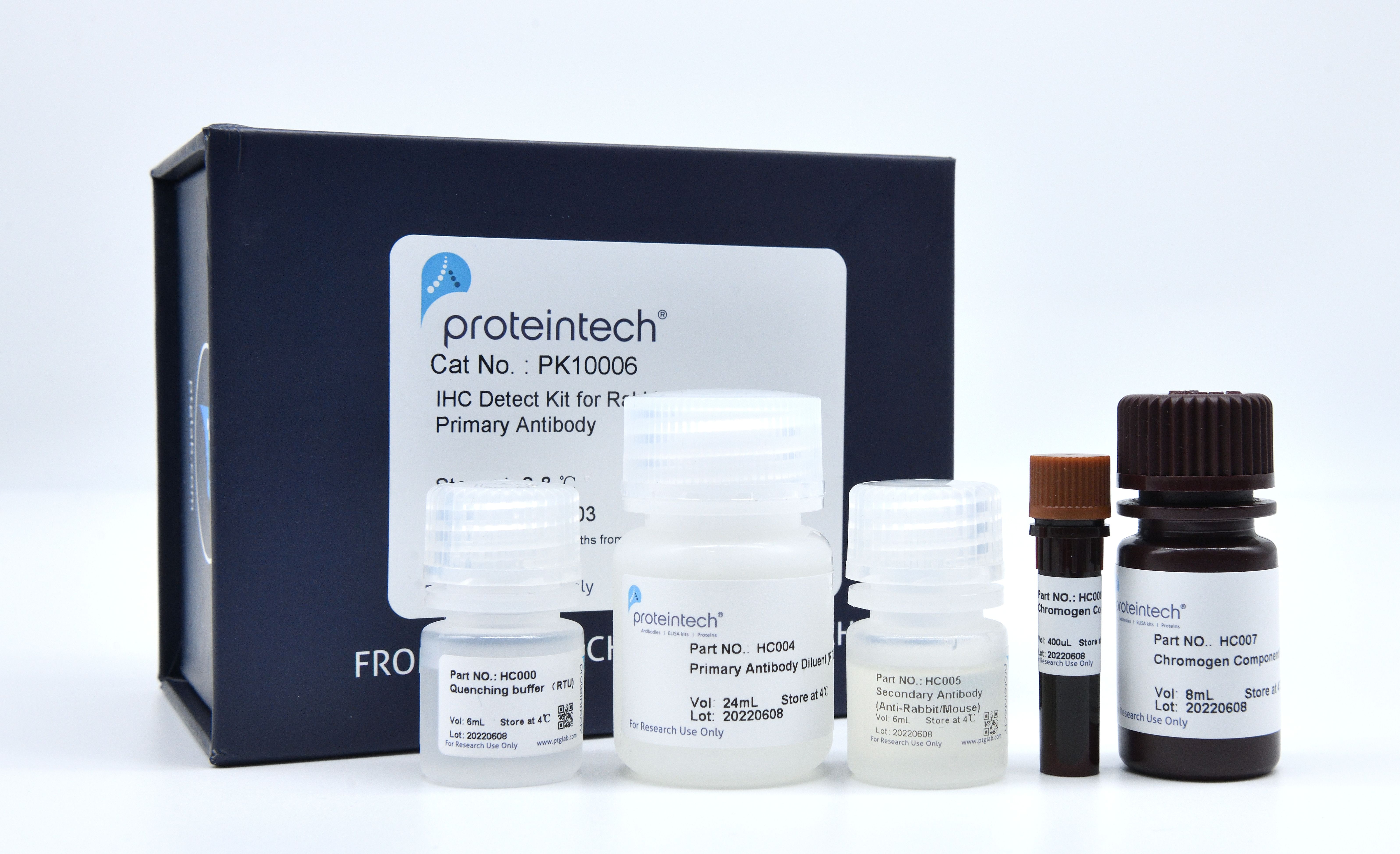
Components
| Part NO. | Component | Size |
| HC000 | Quenching Buffer (RTU) | 6 mL |
| HC004 | Primary Antibody Diluent (RTU) | 24 mL |
| HC005 | Polymer HRP-Goat anti-Rabbit/Mouse Secondary Antibody | 6 mL |
| HC006 | Chromogen Component A | 0.4 mL |
| HC007 | Chromogen Component B | 8 mL |
Size: 60 tests (may vary depending on tissue size).
Storage
All the reagents are stored at 2-8°C. The kit is stable for 6 months from the date of receipt.
Usage
1. Prepare slides from tissues sections following routine methods. Deparaffinize tissues with xylene and re-hydrate using a decreasing ethanol gradient.
2. Perform antigen retrieval following the manufacturer’s recommendations of the primary antibody.
3. Quenching (optional): Based on our experience, endogenous peroxidase quenching is not necessary in most cases and can sometimes even have adverse effects on staining. However, we have included a quenching reagent in this kit since the quenching step is widely recommended in publications.
3.1 Wash the slides with 1x wash buffer. Drain off the liquid on the slides and absorb any residual buffer around the tissue section (making the slides as dry as possible). Use a hydrophobic IHC pen to draw a circle on the glass slides around tissue sections (optional).
3.2 Apply 30-80 uL (depending on the size of the tissue) of the ready-to-use Quenching Buffer (Part NO. HC000) to the slide, covering the entire tissue. Leave the slides at room temperature for 10 minutes. Briefly wash the slides with 1x wash buffer after quenching.
4. Block the tissues with blocking buffer (e.g., 3% BSA in PBS) for 30 minutes at room temperature. Drain the liquid off the slides and absorb the blocking buffer around the tissue section.
* The Primary Antibody Diluent (Part NO. HC004) could also be used as alternative of blocking buffer.
5. Use the Primary Antibody Diluent (Part NO. HC004) in the kit to dilute your primary antibody (ONLY primary antibodies raised in rabbits/mice should be used with this kit). Apply 80-200 uL (depending on the size of the tissue) of the diluted primary antibody to the slide, covering the entire tissue. Incubate at room temperature in a wet box with a lid for 1 hour.
6. Wash the slides three times with 1x wash buffer following primary antibody incubation. Drain the liquid off the slides and absorb any residual buffer around the tissue section. Apply 30-80 uL (depending on the size of the tissue) of the ready-to-use Polymer HRP-Goat anti-Rabbit/Mouse Secondary Antibody (Part NO. HC005) to the slide, covering the entire tissue. Incubate at room temperature in a wet box with a lid for 30 minutes.
7. Wash the slides three times with 1x wash buffer following secondary antibody incubation. Drain the liquid off the slides and absorb any residual buffer around the tissue section. Use pipette to prepare an appropriate volume of Chromogen in a microcentrifuge tube. Ratio of solutions Chromogen Component A (Part NO. HC006) to Chromogen Component B (Part NO. HC007) is 1:20.
* Depending on the size of the tissue sample, approximately 30-80 ul/slide is needed.
Use pipette to add enough Chromogen to cover the tissue. Lay slides flat on the benchtop at room temperature for 5 minutes, or until a brown color develops. If color is visible, stop reaction early by washing with 1x wash buffer.
8. Perform counter staining with hematoxylin.
9. Wash the slides with wash buffer or pure water. Dehydrate tissues using an increasing ethanol gradient followed by xylene. Mount the slides with cover slides and analyze with a light microscope once they are fully dried.
IHC Detect Kit for Rabbit/Mouse Primary Antibody User Manual
Application Note
This kit is recommended for FFPE tissues and not for frozen sections.
Cited in Article as
PK10006, IHC Detect Kit for Rabbit/Mouse Primary Antibody, Proteintech, IL, USA
Publications
| Application | Title |
|---|---|
Bioact Mater Type II collagen scaffolds repair critical-sized osteochondral defects under induced conditions of osteoarthritis in rat knee joints via inhibiting TGF-β-Smad1/5/8 signaling pathway | |
Nat Commun Inhibited peroxidase activity of peroxiredoxin 1 by palmitic acid exacerbates nonalcoholic steatohepatitis in male mice | |
Redox Biol ent-Kaurane diterpenoids induce apoptosis and ferroptosis through targeting redox resetting to overcome cisplatin resistance. | |
JCI Insight A congenital CMV infection model for follow-up studies of neurodevelopmental disorders, neuroimaging abnormalities, and treatment. | |
Cell Rep Roseburia intestinalis sensitizes colorectal cancer to radiotherapy through the butyrate/OR51E1/RALB axis |