Recombinant Human Endoglin/CD105 protein (His Tag)
Species
Human
Purity
>90 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0587
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human Endoglin protein Glu26-Gly586 (Accession# P17813-1) with a His tag at the C-terminus. |
| GeneID | 2022 |
| Accession | P17813-1 |
| PredictedSize | 61.5 kDa |
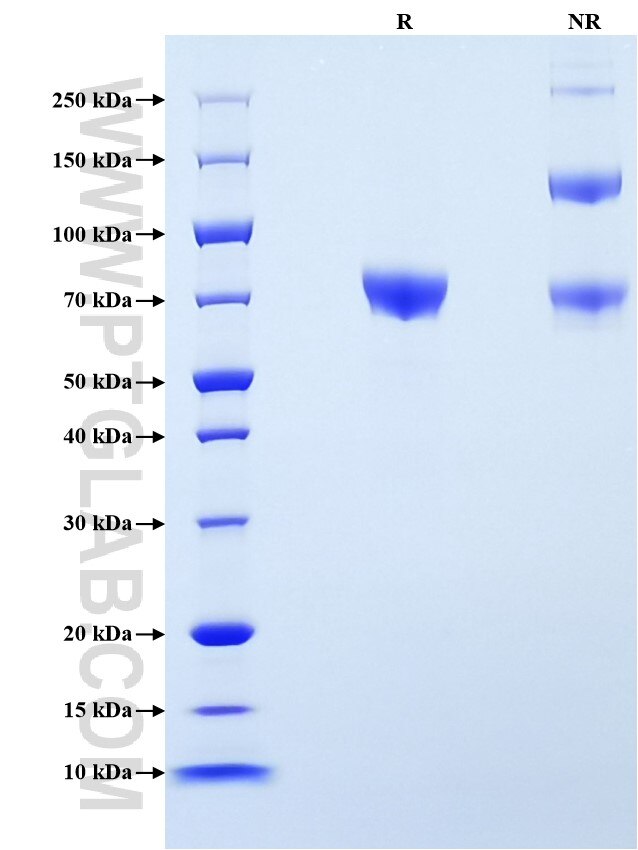
| SDS-PAGE | 65-85 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Endoglin (ENG, CD105) is a homodimeric cell membrane glycoprotein of 180 kDa, composed of disulphide-linked subunits of 90-95 kDa. Endoglin is a proliferation-associated and hypoxia-inducible protein mainly expressed on vascular endothelial cells. It acts as an accessory receptor for transforming growth factor beta (TFG-β) and is involved in vascular development and remodelling. The important role of Endoglin in angiogenesis and in tumor progression makes it an ideal target for antiangiogenic therapy and a good marker for tumor prognosis. The extracellular domain of membrane-bound Endoglin can be proteolytically cleaved, releasing a soluble form of Endoglin (sCD105). Increased levels of sCD105 are linked to the pathogenesis of severe vascular disease, and also correlate with poor prognosis in patients suffering from various types of cancer.
References:
1. Cheifetz S et al. (1992) Journal of Biological Chemistry. 267(27): 19027-19030. 2. Fonsatti E. et al. (2003) Oncogene. 22(42): 6557-6563. 3. Nassiri F. et al. (2011) Anticancer research. 31(6): 2283-2290. 4. Duff S E. et al. (2003) The FASEB Journal. 17(9): 984-992. 5. Pappa C A. et al. (2013) Hematological oncology.31(4): 201-205.