Why is the Fab-Trap™ so Fab-ulous?
ChromoTek’s Fab-Trap™ is an IP reagent based on an antibody Fab-fragment.
Different antibody formats can be used in IP applications, including full-length antibodies, Fab-fragments, and Nanobodies. In this blog, we elaborate on the basic principle and advantages of Fab-fragment based reagents for IP. More simply, we ask “Why is the Fab-Trap™ Fab-ulous?”
What is a Fab-Trap™?
Immunoprecipitation (IP) is a technique for capturing a protein out of a solution using an antibody that specifically binds to the protein antigen. Several different antibody formats can be used in IP applications, including full-length antibodies, Fab-fragments, and Nanobodies. Our “Advantages and limitations of different antibody formats in immunoprecipitation” blog contains further information on the differences and advantages of each format. One of the formats discussed is ChromoTek’s Fab-Trap™, an IP reagent based on an antibody Fab-fragment.
The typical Y-shaped structure of bivalent antibodies such as immunoglobulin G (IgG) can be divided into two major domains, the Fc-fragment and the Fab-fragment. The Fab-fragment includes the antigen-binding arm and consists of the light chain VL and CL domains and the heavy chain VH and CH1 domains. The light and heavy chain parts of the Fab-fragment are each around 25 kDa and are linked by a disulfide bridge.
The Fab-Trap™ is an IP reagent based on a Fab-fragment of a conventional antibody that is covalently coupled to Agarose beads. Fab-Traps™ offer several advantages in IP compared to conventional IgGs bound to beads.
We highlight several benefits in the following section.
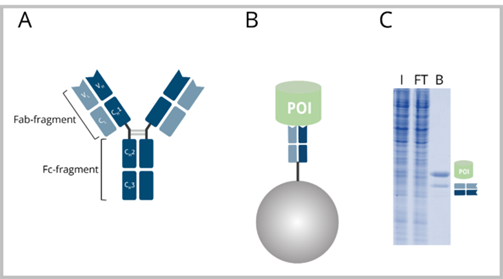
A: Antibody with heavy chain domains VH, CH1, CH2, CH3 and light chain domains VL and CL. B: Fab-Trap™: Fab-fragment coupled to beads with bound POI. C: SDS-PAGE of an exemplary IP reaction performed with a Fab-Trap™. I: Input, FT: Flow-Through, B: Bound. The B fraction contains the POI as well as the Fab-fragment heavy and light chain.
Minimal contamination by the Fab-Trap™ reagent on SDS-PAGE and WB
The binding protein of a Fab-Trap™, the Fab-fragment consists of two protein chains of only ~25 kDa each. Therefore, although the Fab-fragment is eluted during denaturation with SDS-sample buffer, only one band is visible at around 25 kDa on subsequent SDS-PAGE and Western blot analyses. In contrast, elution of a full-length antibody results in two bands at ~25 and ~50 kDa, respectively. Since there is no contamination in SDS-PAGE and WB above 25 kDa, IP results are cleaner and easier to interpret using the Fab-Trap™.
Please note that it is possible to avoid contamination by the Fab-fragment in downstream analyses when using milder elution procedures such as elution with low pH or competitive peptide elution.
Lower background on SDS-PAGE by the host-cell proteins
The Fab-fragment is only a third of the size of a conventional antibody. Due to its smaller size, the Fab-fragment offers less surface and thus minimizes unwanted interactions with the host-cell proteome. This results in IP with low background and reduces the chances of identifying false positive interactions.
Harsh washing conditions avoid unspecific binding of cellular proteins
The Fab-fragment tolerates harsher wash buffers compared to conventional antibodies, thereby enabling the effective removal of unwanted cellular proteins by stringent washing steps. Combined with the low unspecific binding of cellular proteins to the Fab-fragment itself, the Fab-Trap™ enables IP with superior purity and less background.
Fewer contaminations in mass spectrometry data
On-bead digestion is a time-effective and efficient method of preparing IP samples for subsequent mass spectrometric (MS) analysis. Due to the reduced size of the Fab-fragment compared to a conventional antibody, you will find fewer antibody derived peptide contaminations in on-bead digested MS samples, thereby facilitating detection of your protein of interest and putative interaction partners.
Ready-to-use reagent saves pre-incubation time
The Fab-fragment in the Fab-Trap™ comes pre-conjugated to Agarose beads. This eliminates the pre-incubation time for protein A or G beads that is required for IP with regular antibodies. Faster IP also facilitates the pull-down of unstable proteins or complexes. Moreover, there is no need for pre-clearing with protein A or G beads.
Broader range of target organisms and cells
There are several examples of proteins that bind to the Fc-fragment of antibodies, such as Fc receptors from immune cells and protein A or G from certain bacteria such as Staphylococcus aureus. The Fab-Trap™ lacks the Fc-fragment and thus enables convenient IP with low background from cells that produce Fc binding proteins, with no need for tedious pre-clearing steps.
Recombinant Fab-fragments for higher reliability and reproducibility
The Fab-fragments used in our Fab-Traps™ are produced recombinantly. This enables an unlimited supply with virtually no lot-to-lot variations and ensures reliable and reproducible results. In contrast, different antibody batches can vary greatly and may require new optimization of IP experiments.
Summary
The Fab-Trap™ is an excellent alternative to IP with regular antibodies. Due to the smaller size of the Fab-fragment, the Fab-Trap™ offers IP with superior purity, minimized background, and less antibody derived contamination in downstream applications. Moreover, IP can be achieved faster and from a wider range of target organisms.
Curious about the Fab-ulous Fab-Trap™? Test our DYKDDDDK Fab-Trap™
