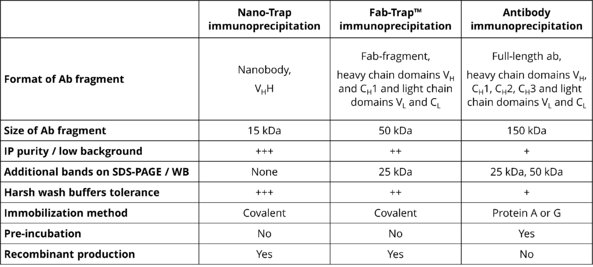
Advantages and limitations of different antibody formats in immunoprecipitation
Immunoprecipitation (IP) is a technique used to isolate a protein from a cellular extract. The protein of interest (POI) is recognized by a specific antibody (Ab) conjugated to beads. Different antibody formats can be used in IP, e.g., a full-length Ab, a Fab-fragment, or a Nanobody. Below, we discuss the advantages and limitations of the different Ab formats.
What is immunoprecipitation?
During IP a POI is enriched from a cellular extract by an antibody conjugated to beads. Unbound, or nonspecifically bound cellular content, such as other proteins, cell debris, or lipids, is removed by washing. The POI remains bound to the beads and is subsequently eluted with SDS sample buffer or with acidic buffer. The IP results are analyzed on SDS-PAGE and Western blot (WB).
IP with a full-length antibody
Conventional Abs used for IP are typically monoclonal or polyclonal immunoglobulin G (IgG) isolated from rabbit or mouse. They are ~150 kDa in size and composed of two heavy (2x ~50 kDa) and two light (2x ~25 kDa) chains. The most common and straightforward option for immobilizing Abs on beads for IP is to bind the Ab via its Fc-fragment to protein A or G beads. When using protein A or G beads, either the cell lysate is first incubated with the Ab and then the beads are added, or the Ab is pre-loaded onto the beads, which are then added to the cell lysate. Analysis of the IP on SDS-PAGE and WB show additionally to the POI also the heavy and light chain of the Ab.
Advantages:
- During immobilization of the Ab on protein A or G, the paratope (binding region) of the Ab is usually neither modified, unfolded, nor denatured and the Ab remains intact.
Limitations:
- The heavy and light chains of the Ab are visible on SDS-PAGE and WB; this complicates identification or can mask the POI and its interaction partners.
- Abs are sensitive to harsh buffer ingredients such as detergents, reducing agents, and salts, which may lead to the release of the POI during washing steps.
- Protein A or G and the large Ab molecule offer more surface for unwanted interactions with the cellular proteome or other contaminants, which increases the background of the IP.
- Immobilization of the Ab on protein A or G beads involves two incubation steps; these are time-consuming and require optimization and greater hands-on time.
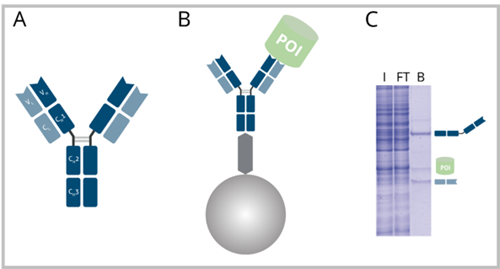
A: Ab with heavy chain domains VH, CH1, CH2, CH3 and light chain domains VL and CL. B: Ab coupled to protein A or G beads with bound POI. C: SDS-PAGE of an exemplary IP reaction performed with a regular Ab. I: Input, FT: Flow-Through, B: Bound. The B fraction contains the POI as well as the Ab heavy and light chain.
IP with a Fab-fragment
Fab-fragments are derived from monoclonal Abs from mouse or rabbit. They are composed of the light chain (~25 kDa) as well as the VH and CH1 domain of the heavy chain (~25 kDa), making ~50 kDa in total. ChromoTek’s Fab-Trap™ is an IP reagent that consists of a Fab-fragment, covalently bound to beads. Despite the short equilibration, no additional pre-incubation steps are required in the preparation procedure of the Fab-Trap™. Also, the Fab-fragment is co-eluted upon boiling in SDS sample buffer. However, due to the reduced size of the Fab-fragment, only one additional band at ~25 kDa is visible on subsequent SDS-PAGE and WB. The band is composed of the light chain as well as the heavy chain fragment.
Advantages:
- Due to the lack of the Fc-fragment, the Fab-Trap™ generates fewer contaminating Ab bands on SDS-PAGE and WB and no bands above ~25 kDa. Elution of the Fab-fragment can be avoided by applying milder elution conditions such as low pH or peptide elution.
- Compared to full-size Abs, the Fab-Trap™ tolerates increased concentrations of detergents and reducing agents, which enables more stringent washing.
- Due to the smaller size of the Fab-fragment and the lack of protein A or G, the Fab-Trap™ provides less surface for unwanted interactions. This leads to lower background compared to IP with full-size Abs.
- The Fab-Trap™ is ready to use and requires less hands-on time and no incubation with protein A or G beads.
- The Fab-Trap™ is produced recombinantly, which results in virtually no lot-to-lot variations and higher reproducibility.
Limitations:
- The additional band on SDS-PAGE and WB at ~25 kDa can mask the POI and interaction partners.
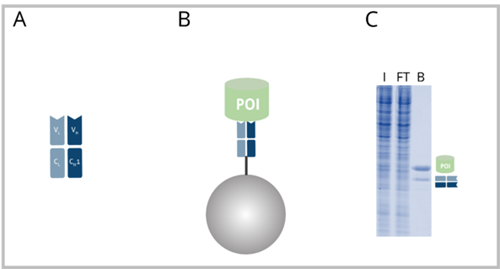
A: Fab-fragment with heavy chain domains VH and CH1 and light chain domains VL and CL. B: Fab-fragment coupled to beads (Fab-Trap™) with bound POI. C: SDS-PAGE of an exemplary IP reaction performed with a Fab-Trap™. I: Input, FT: Flow-Through, B: Bound. The B fraction contains the POI as well as the Fab-fragment heavy and light chain.
IP with a Nanobody
Nanobodies are single-domain antibody fragments derived from alpaca heavy chain only antibodies. They consist of the VH-domain of the heavy chain (also called VHH) and are around 15 kDa in size. ChromoTek’s Nano-Trap is an IP reagent that consists of a Nanobody, covalently bound to beads. Nano-Traps are ready to use and require only a short equilibration step, with no need for additional pre-incubation, e.g., with protein A or G beads. During elution, the Nanobody of the Nano-Trap remains bound to the beads and does not appear in SDS-PAGE or WB analyses.
Advantages:
- On SDS-PAGE and WB only the POI band is visible, with no contamination of the Nano-Trap.
- Nano-Traps offer the highest purity for IP as Nanobodies are the smallest molecules and bind with high specificity.
- Nano-Traps can withstand very high concentrations of detergents, reducing agents, and salts, enabling the highest stringency during washing and IP even under denaturing conditions. Therefore, Nano-Traps offer the lowest background among IP reagents.
- Nano-Traps are ready to use, do not need to be pre-incubated, and therefore require less hands-on time.
- Due to the recombinant production of the Nanobodies, a limitless supply of Nano-Traps is possible, with no lot-to-lot variation, leading to more reproducible results.
Limitations:
- Due to their preference for native and structurally intact epitopes, the binding of Nano-Traps to fixed samples may be challenging.
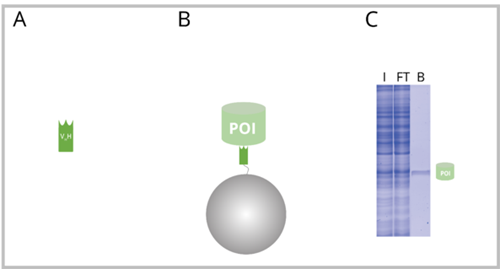
A: Nanobody consisting of the alpaca heavy chain antibody domain VHH. B: Nanobody coupled to beads (Nano-Trap) with bound POI. C: SDS-PAGE of an exemplary IP reaction performed with a Nano-Trap. I: Input, FT: Flow-Through, B: Bound. The B fraction contains only the POI.
Summary
The advantages and limitations of the different antibody formats are summarized in the table below. Taken together, Nano-Traps offer the best performance for IP. ChromoTek offers a broad portfolio of Nano-Traps against common proteins (e.g., GFP, RFP, Halo) as well as peptide tags (e.g., Myc, V5, Spot). The Fab-Trap™ is an excellent alternative for IP when a Nano-Trap is not available for the POI. Although Abs have several limitations compared to the other IP formats, they are often considered when neither Nano-Traps nor Fab-Traps™ are offered for the POI and are particularly used when endogenous proteins are precipitated without tags.