What do you get when you buy a GMP-grade product?
GMP Manufacturing, benefits, and ISO certifications explained
In 2020, Proteintech received ISO 13485 certification and now offers GMP-grade products. What does that certification mean? ‘ISO’ stands for International Organization for Standardization. Formed in 1943, ISO is a non-governmental body charged with promoting worldwide standards to ensure safe, reliable, and high-quality products. ‘13485’ refers to the specific certification for medical devices and ancillary products, such as cytokines. 'GMP' stands for Good Manufacturing Practice, which refers to the quality control procedures set out by agencies that control the authorization of pharmaceutical and medical products. Therefore, a GMP-certified protein is one that can be used in cell and gene therapies.
The heart of this certification is the quality management system (QMS). Informally, this can be thought of as a company-wide instruction manual for reproducibility and continual improvement. More formally, it is an internal system to document standardized company practices for processes, procedures, and responsibilities to ensure product safety and quality. It outlines everything from manufacturing guidelines to handling customer complaints.
The ISO 13485 certification, therefore, ensures quality, consistency, and traceability. Combined with the advantages of the human cell-expressed system, HumanKine® GMP-grade proteins are ideal for use in cell and gene therapy manufacturing.
5 Highlights of GMP-Grade HumanKine Products
1. Lot consistency
A key part of the QMS is documentation on how to perform each step, from buying raw materials to packaging the final product. Besides detailed procedures, QMS outlines rigorous training to ensure laboratory personnel consistently follow the same production protocols. These documentation and training practices limit the sources of variability, resulting in high lot reproducibility.
 |
|
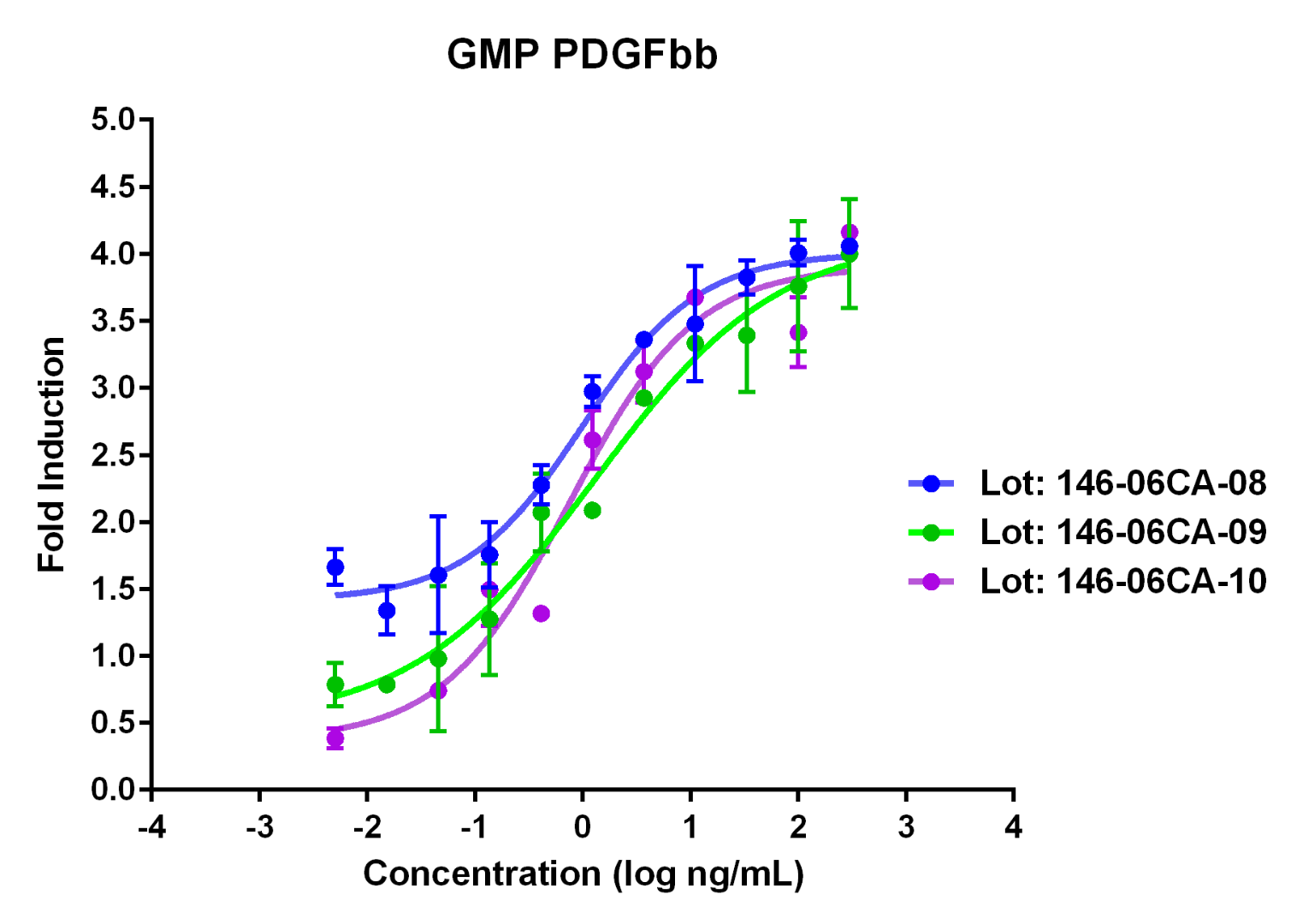
Lot-to-lot comparison of GMP-grade Animal-free Recombinant human PDGFbb (Catalog: HZ-1308). This protein stimulates dose-dependent proliferation of the NIH/3T3 mouse fibroblast cell line. Viable cell number was quantitatively assessed by PrestoBlue Cell Viability Reagent. NIH/3T3 cells were plated and serum starved with 0.1% FBS for 24 hours and increasing concentrations of recombinant human PDGFbb were added for 48 hours before the addition of PrestoBlue Cell Viability Reagent. EC50 is less than 5 ng/mL as determined by a 4-parameter non-linear regression model. |
2. Lot traceability
An advantage of the extensive documentation in the QMS is traceability. Each step in the manufacturing process requires documentation of who performed the work and what was done. Thus, the manufacturer can provide documentation of the entire process to customers so that they can verify that the work was done to their exact specifications. Additionally, if a customer files a complaint, the issue can be pinpointed by examining the documentation.
3. Tag-free
Purification tags are often used to isolate proteins. Unfortunately, these tags often change the structure of a protein of interest. This, in turn, may interfere with the active site of the protein resulting in altered biological activity. Additionally, the presence of a tag may increase the immunogenicity of some proteins. This may cause issues if the protein is part of a therapeutic. To avoid these issues, HumanKine® proteins are produced without tags and purified using biophysical methods. Many non-GMP proteins will be affinity purified and have residual tags so are not suitable to use in GMP manufacturing even with additional testing and risk assessments.
4. Animal component-free and endotoxin-free
Material originating from a foreign organism in a therapeutic creates the risk of medical complications. One of the most dangerous molecules is endotoxin, a bacterial lipopolysaccharide that can cause sepsis. To mitigate xenobiotic risk, HumanKine® products are made without any raw material from foreign organisms. Additionally, the use of human cells limits endotoxin levels in the final product and each lot is tested for endotoxin contamination, making it an ideal expression system for GMP-grade proteins.
5. Ready-to-use products in your GMP process
The ISO 13485 certification means that all the bioburden testing and risk assessments, which would be required to use an RUO product in your process, have already been completed, saving precious time and resources. You have the assurance that these GMP proteins can be used in your process without risk.
Related Content
cGMP Grade Cytokines & Growth Factors
Cytokines and Growth Factors: Why you should give a HEK
FGF Basic-TS: No media changes required over the weekend
Why human cell expressed proteins are superior for clinical applications
Considerations when choosing recombinant proteins for your research applications
HumanKine® Growth Factors are the perfect tool for your cerebral organoid research
Human serum albumin’s importance in biology and biotech

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
