The Gut Microbiome and Depression: Current Insights and Future Directions
Written by Hasnain Methiwala, PhD Student at the University of Minnesota Medical School
Depression, or Major Depressive Disorder (MDD), is a widespread and debilitating psychiatric condition that significantly impacts individuals and society at large by reducing quality of life, lost productivity, and increased healthcare costs. Globally, over 300 million people suffer from depression (WHO 2023), including approximately 21 million adults in the U.S., accounting for 8.3% of the U.S. population (Evans-Lacko, Aguilar-Gaxiola et al. 2018, NIMH 2025). This makes MDD one of the leading causes of disability in the country. Alarmingly, despite its high prevalence, only about 41% of U.S. adolescents receive treatment within a given year (NIMH 2025). The complexity and heterogeneity of MDD continue to challenge our understanding of its underlying mechanisms, leaving effective and targeted therapeutic strategies largely elusive.
Gut Microbiota and CNS
There has been considerable research investigating the role of gut microbiota in modulating the mechanisms and function of the central nervous system (CNS). This connection was first systematically explored in a landmark article by Aziz and Thompson, who built upon early physiological observations to demonstrate how emotional states can directly influence gastrointestinal (GI) function, thereby establishing a foundational link between the brain and the gut (Aziz and Thompson 1998). Their work highlighted the importance of the gut-brain axis and set the stage for modern studies examining how gut microbiota can influence neurodevelopment, behavior, memory, cognition, and mood through bidirectional communication pathways (Mayer 2011; Bangsgaard Bendtsen, Krych et al. 2012; Rogers, Keating et al. 2016; Kelly, Minuto et al. 2017; Kim and de La Serre 2018). Extensive research sparked by this has resulted in the accumulation of evidence revealing a larger role that the gut microbiota plays in mediating emotional states and the pathology of psychiatric disorders (McGuinness, Loughman et al. 2024). Many studies have reported that patients with gastrointestinal disorders, including irritable bowel syndrome and inflammatory bowel disease, have a higher risk of mental health disorders, indicating the importance of the gut-brain axis in making one susceptible to mood disorders (Hu, Chen et al. 2021; Staudacher, Black et al. 2023).
This has also provided a new frontier for investigating psychiatric disorders with germ-free (GF) animals through aseptic reproduction and aseptic feeding, becoming a new animal model type. The mice are devoid of any microorganisms in their body, thus allowing researchers to isolate the effects of specific gut microbes on host physiology (Al-Asmakh and Zadjali 2015). Preclinical and clinical studies have highlighted that phenotypical and genotypical changes in the gut microbiome, known as dysbiosis, can significantly alter the onset and progression of depression via regulating the gut-brain axis (Yu, Jia et al. 2017; Zu, Xin et al. 2024; Patel, Panche et al. 2025). For instance, studies have demonstrated the bidirectional relationship between gut dysbiosis and depression, showing that not only can depression alter the composition of the gut microbiome, but also that microbiota-targeted therapies can help alleviate depression-like symptoms (Bravo, Forsythe et al. 2011; Yano, Yu et al. 2015; Zhou and Foster 2015; Wong, Inserra et al. 2016). Furthermore, preclinical and clinical studies of fecal transplantation also showed promising results in enhancing neurochemical functions and reducing inflammatory phenotypes in mouse models of depression (Cai, Shi et al. 2019; Li, Wang et al. 2019; Doll, Vazquez-Castellanos et al. 2022; Zhang, Bi et al. 2024).
Mechanisms of Gut Microbiota in Depression
-
The Hypothalamus Pituitary Gut Brain Axis
The Hypothalamus Pituitary Adrenal (HPA) axis plays a central role in regulating the body’s stress and mood response, and it is important in understanding the underlying mechanisms of mood disorders like MDD (Baumeister, Lightman et al. 2014). Research in mouse models of depression, such as chronic restraint stress, has shown that prolonged stress can desensitize the HPA axis, diminishing its responsiveness to key stress hormones like adrenocorticotropic hormone (ACTH) and corticosterone (CORT) (Lightman, Birnie et al. 2020), with excess CORT exposure shown to increase immune activation in microglial cells in the brain (Xu, Lang et al. 2019). Studies with germ-free mice have shown that alterations in gut microbiota diversity led to a disrupted HPA axis response, while microbial metabolites that act as signaling molecules influence the development and functioning of the central nervous system (CNS) (Ikegami, Narabayashi et al. 2024). Furthermore, reports also confirmed that a change in the homeostasis of the gut microbiota because of poor diet, chronic stress, and antibiotic use can further exacerbate the symptoms associated with MDD (Yang, Li et al. 2021).
-
Neuro-Entero-Endocrine Pathways
The metabolism of nutrients by gut microbiota is closely linked to nutrient-sensing cells and the secretion of peptides by enteroendocrine cells and gut bacteria in the intestine (Duca and Lam 2014). Bioactive peptides released by the microbiota can influence the gut-brain axis, playing a modulatory role in neural signaling (Holzer and Farzi 2014; Buey, Layunta et al. 2023). Studies using bioactive peptides showed prevention of memory impairment in mice by improving autophagy, as shown by increases in Akt and LC3-II protein function in the hippocampus (Lin, Li et al. 2024). Moreover, the diverse composition of gut microbiota contributes to the production of various neuroactive substances. Studies with treatment with GABA and acetylcholine-producing Lactobacillus and Bifidobacterium species showed an increase in protein expression of synaptic integrity proteins like PSD-95 and neuropeptides BDNF in the hippocampus of mice exposed to chronic stress (Zhao, Chen et al. 2023; Xu, Li et al. 2025). Reports have also shown beneficial effects of Escherichia, Candida, and Enterococcus species in serotonin and Bacillus, and Saccharomyces in dopamine and noradrenaline productions in the gut (Yano, Yu et al. 2015; Feng, Meng et al. 2023). Alterations in both the composition and population diversity of these bacteria have been linked to depression and mood disorders.
-
Immunological Mechanisms
In recent years, a growing body of research has supported the theory that inflammation plays a central role in the pathogenesis of psychiatric disorders. Elevated levels of circulating cytokines have been linked to disruptions in neurohormonal and neurochemical balance, leading to mood disorders (Raison, Capuron et al. 2006). One of the key contributors to this inflammatory process is known as leaky gut syndrome (LGS), which is thought to arise from disturbances in the intestinal microbiota. Such disturbances compromise the integrity of the intestinal barrier—normally maintained by tight junctions between enterocytes—leading to increased permeability. This allows toxic bioactive molecules, including lipopolysaccharides (LPS) from gram-negative bacteria, to enter systemic circulation (Makris, Karianaki et al. 2021). Therapeutic interventions targeting the gut microbiota have been shown to restore barrier function by upregulating tight junction proteins such as Occludin, Claudin-1, and ZO-1 (Zhang, Wang et al. 2020). Many studies have reported that psychiatric disorders such as depression and anxiety are associated with increased inflammatory markers in the brain, with stress-induced inflammatory profiles resembling those triggered by pathogenic infections (Gaspersz, Lamers et al. 2017; Lamers, Milaneschi et al. 2019; Peirce and Alviña 2019; Hu, Lu et al. 2022; Min, Wang et al. 2023; Mac Giollabhui, Slaney et al. 2025). This heightened inflammation can compromise the integrity of the blood-brain barrier (BBB) (Braniste, Al-Asmakh et al. 2014), which normally regulates and protects the brain’s internal environment (Engelhardt 2003; Abbott, Patabendige et al. 2010). Metabolites produced by the gut microbiota, such as short-chain fatty acids including butyrate and acetate, as well as bacterial components like lipopolysaccharide (LPS), can gain access to the central nervous system when the gut barrier is compromised (Bien-Ly and Watts 2014; Inaba, Yamashiro et al. 2023). This exposure can disrupt the expression and proper assembly of tight junction proteins, which are critical for maintaining the structural and functional integrity of the blood-brain barrier (BBB) (Bhattarai 2018). An imbalanced gut microbiota and sustained inflammatory states can further contribute to a ‘leaky’ BBB, allowing harmful substances, including bacterial metabolites, to enter the central nervous system (Virgintino, Errede et al. 2004; Bien-Ly and Watts 2014; Braniste, Al-Asmakh et al. 2014; Logsdon, Erickson et al. 2018). This exposure can exacerbate pathological processes, promoting the development and progression of disorders such as depression and anxiety. Additionally, studies have reported elevated levels of inflammatory biomarkers in both microglia and peripheral immune cells, including increased expression of CD68, IL-1β, TNF-α, and IL-6, alongside reduced levels of the anti-inflammatory cytokine IL-10 (Tung, Lai et al. 2023). This inflammatory environment is believed to contribute to the activation of cytokine release, thereby promoting a pro-inflammatory state implicated in the development of mood disorders.
-
Bacterial Metabolites
Microorganisms in the gut metabolize various dietary nutrients and intestinal components to produce a diverse range of bioactive compounds. These metabolites not only support gut health but also play a profound role in influencing overall host physiology and disease states. Among the most studied are short-chain fatty acids (SCFAs), lactate, tryptophan metabolites, and neuroactive amino acids (Rowland, Gibson et al. 2018). SCFAs, primarily butyrate, propionate, and acetate, are involved in regulating pH, enhancing mucus production, supporting epithelial integrity, and modulating immune responses. They are also key players in gut-brain communication, primarily through interactions with enteroendocrine cells that relay signals along the gut-brain axis (Silva, Bernardi et al. 2020). Studies with chronically stressed animals showed a dysfunction in lactate metabolism by gut bacteria, leading to a reduction in Sphingosin-1-phosphat receptor 2 (S1PR2) and increased TNF-alpha, resulting in increased vulnerability to stress (Shan, Ai et al. 2020). Lactate can cross the blood–brain barrier and contributes to brain energy metabolism while modulating neuronal activity. Tryptophan metabolites, on the other hand, impact serotonin synthesis, influencing peripheral serotonin levels and potentially playing a central role in mood regulation (O’Mahony, Clarke et al. 2015). Altogether, the composition and activity of these metabolites form a critical biochemical profile that may contribute to the onset, progression, and treatment of depression.
-
Microbiota-Microglial Activation
Studies have shown the importance of microglia in various psychiatric disorders, with stress resulting in increased microglial activation as reported by an increase in IBA-1 and GFAP in the hippocampus of mice exposed to stressful stimuli (Xu, Lang et al. 2019). In addition to microbial metabolites, the release of neurotransmitters and neuropeptides has been shown to influence microglial activation and function. RNA sequencing analyses of microglia from germ-free mice reveal altered gene expression patterns and abnormal phenotypes, indicating a compromised ability to respond to immune challenges in the CNS (Huang, Wu et al. 2023; Gui, Liu et al. 2025). Such dysfunctional microglial profiles have been consistently observed in animal models of depression (Hao, Ma et al. 2024), suggesting that disrupted regulation of microglial activity by the gut microbiota may play a key role in the development of psychiatric disorders such as depression and schizophrenia.
Therapeutic Strategies Involving Gut Microbiota
Research on the gut microbiome in depression has paved the way for novel therapeutic strategies, including the emergence of “Psychobiotics” as a potential class of treatment for major depressive disorder (MDD). Behavioral studies have demonstrated that probiotic interventions can effectively reduce depression-like behaviors in animal models (Zhang, Wang et al. 2020; Feng, Meng et al. 2023). For instance, supplementation with Lactobacillus helveticus has been shown to alleviate the transgenerational impact of stress in infant rats (Cowan, Callaghan et al. 2016; Wang, Hu et al. 2024). Probiotics have also been found to restore intestinal barrier integrity, offering a direct approach to address the “leaky gut” hypothesis underlying certain mood disorders (Ait-Belgnaoui, Durand et al. 2012). Moreover, probiotic supplementation has been associated with the normalization of stress-induced dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. Clinical studies further support these findings, with both prebiotic and probiotic treatments leading to improvements in depressive symptom severity, reductions in cortisol levels, and alleviation of associated symptoms such as anxiety and insomnia (Liu, Walsh et al. 2019).
Future Implications and Conclusion.
Major Depressive Disorder (MDD) continues to pose a significant global health burden, impacting individuals and society through increased disability, rising healthcare demands, and reduced productivity. While conventional treatments offer relief for some, they often fall short of addressing the broader, multifaceted nature of the disorder. A growing body of evidence from both animal and human studies points to gut microbiota as a key player in the development and progression of MDD. Pathways involving microglial activation, neurotransmitter regulation, intestinal barrier integrity, and HPA axis function provide critical insights into how disturbances in gut health can influence brain function and emotional well-being. These findings not only enhance our understanding of the underlying biology of depression but also emphasize the need for integrative research approaches that bridge neuroscience, microbiology, and psychiatry. Looking forward, modulating the gut microbiome holds promise as a non-invasive and comprehensive strategy for developing more personalized and effective treatments for depression.
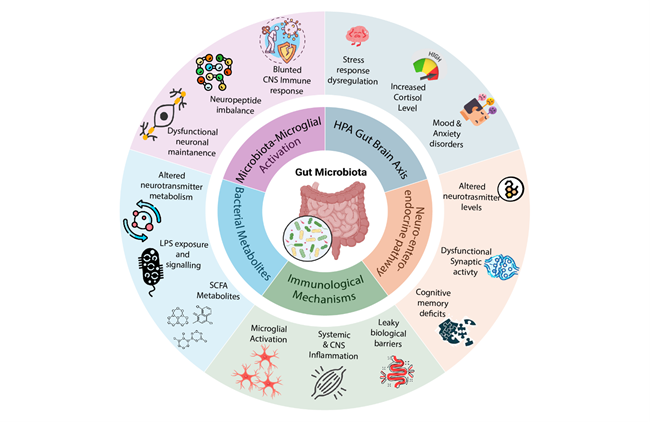
Figure 1: Gut microbiota–brain communication pathways and downstream effects. The gut microbiota regulates brain function and behavior through multiple interconnected mechanisms. The HPA gut–brain axis mediates stress response regulation, with chronic dysregulation leading to elevated corticosterone levels and vulnerability to mood and anxiety disorders. The neuro-enteric-endocrine pathway links microbial activity to altered neurotransmitter levels, synaptic activity, and cognitive outcomes. Immunological mechanisms involve increased gut permeability, systemic and CNS inflammation, and leaky blood–brain barrier function. Bacterial metabolites, including SCFAs, tryptophan derivatives, and LPS, influence neuronal maintenance, signaling, and metabolic homeostasis. Finally, microbiota–microglial activation highlights the role of gut microbes in shaping microglial function, with stress-induced activation and immune dysregulation contributing to psychiatric disorders. Collectively, these pathways underscore the complex bidirectional communication between the gut microbiota and the central nervous system.
Adapted from (Methiwala, Vaidya et al. 2021)
References
-
Abbott, N. J., A. A. K. Patabendige, D. E. M. Dolman, S. R. Yusof, and D. J. Begley (2010). "Structure and function of the blood–brain barrier." Neurobiology of Disease 37(1): 13-25.
-
Ait-Belgnaoui, A., H. Durand, C. Cartier, G. Chaumaz, H. Eutamene, L. Ferrier, E. Houdeau, J. Fioramonti, L. Bueno, and V. Theodorou (2012). "Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats." Psychoneuroendocrinology 37(11): 1885-1895.
-
Al-Asmakh, M. and F. Zadjali (2015). "Use of germ-free animal models in microbiota-related research." J Microbiol Biotechnol 25(10): 1583-1588.
-
Aziz, Q. and D. G. Thompson (1998). "Brain-gut axis in health and disease." Gastroenterology 114(3): 559-578.
-
Bangsgaard Bendtsen, K. M., L. Krych, D. B. Sørensen, W. Pang, D. S. Nielsen, K. Josefsen, L. H. Hansen, S. J. Sørensen and A. K. Hansen (2012). "Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse." PLoS One: 2012;2017(2010):e46231.
-
Baumeister, D., S. L. Lightman, and C. M. Pariante (2014). "The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness." Behavioral Neurobiology of Stress-related Disorders. C. M. Pariante and M. D. Lapiz-Bluhm. Berlin, Heidelberg, Springer Berlin Heidelberg: 13-24.
-
Bhattarai, Y. (2018). "Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers." Neurogastroenterology & Motility 30(6): e13366.
-
Bien-Ly, N. and R. J. Watts (2014). "The Blood-Brain Barrier’s Gut Check." Science Translational Medicine 6(263): 263fs246.
-
Braniste, V., M. Al-Asmakh, C. Kowal, F. Anuar, A. Abbaspour, M. Tóth, A. Korecka, N. Bakocevic, L. G. Ng, P. Kundu, B. Gulyás, C. Halldin, K. Hultenby, H. Nilsson, H. Hebert, B. T. Volpe, B. Diamond, and S. Pettersson (2014). "The gut microbiota influences blood-brain barrier permeability in mice." Science Translational Medicine 6(263): 263ra158.
-
Bravo, J. A., P. Forsythe, M. V. Chew, E. Escaravage, H. M. Savignac, T. G. Dinan, J. Bienenstock, and J. F. Cryan (2011). "Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve." Proceedings of the National Academy of Sciences 108(38): 16050-16055.
-
Buey, B., E. Layunta, E. Latorre, and J. E. Mesonero (2023). "Potential role of milk bioactive peptides on the serotonergic system and the gut-brain axis." International Dairy Journal 137: 105534.
-
Cai, T., X. Shi, L.-Z. Yuan, D. Tang, and F. Wang (2019). "Fecal microbiota transplantation in an elderly patient with mental depression." International Psychogeriatrics 31(10): 1525-1526.
-
Cowan, C. S. M., B. L. Callaghan, and R. Richardson (2016). "The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats." Translational Psychiatry 6(5): e823.
-
Doll, J. P., J. F. Vazquez-Castellanos, A.-C. Schaub, N. Schweinfurth, C. Kettelhack, E. Schneider, G. Yamanbaeva, L. Mählmann, S. Brand, and C. Beglinger (2022). "Fecal microbiota transplantation (FMT) as an adjunctive therapy for depression—case report." Frontiers in Psychiatry 13: 815422.
-
Duca, F. A. and T. K. T. Lam (2014). "Gut microbiota, nutrient sensing and energy balance." Diabetes, Obesity and Metabolism 16(S1): 68-76.
-
Engelhardt, B. (2003). "Development of the blood-brain barrier." Cell and Tissue Research 314(1): 119-129.
-
Evans-Lacko, S., S. Aguilar-Gaxiola, A. Al-Hamzawi, J. Alonso, C. Benjet, R. Bruffaerts, W.-T. Chiu, S. Florescu, G. de Girolamo, and O. Gureje (2018). "Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: results from the WHO World Mental Health (WMH) surveys." Psychological Medicine 48(9): 1560-1571.
-
Feng, S., C. Meng, Y. Liu, Y. Yi, A. Liang, Y. Zhang, and Z. Hao (2023). "Bacillus licheniformis prevents and reduces anxiety-like and depression-like behaviours." Applied Microbiology and Biotechnology 107(13): 4355-4368.
-
Gaspersz, R., F. Lamers, G. Wittenberg, A. T. F. Beekman, A. M. van Hemert, R. A. Schoevers, and B. W. J. H. Penninx (2017). "The role of anxious distress in immune dysregulation in patients with major depressive disorder." Translational Psychiatry 7(12): 1268.
-
Gui, S., Y. Liu, J. Pu, D. Wang, X. Zhong, W. Chen, X. Chen, Y. Chen, X. Chen, W. Tao, and P. Xie (2025). "Systematical Comparison Reveals Distinct Brain Transcriptomic Characteristics in Depression Models Induced by Gut Microbiota Dysbiosis and Chronic Stress." Molecular Neurobiology 62(6): 7957-7974.
-
Hao, W., Q. Ma, L. Wang, N. Yuan, H. Gan, L. He, X. Li, J. Huang, and J. Chen (2024). "Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3." Microbiome 12(1): 34.
-
Holzer, P. and A. Farzi (2014). "Neuropeptides and the Microbiota-Gut-Brain Axis." Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. M. Lyte and J. F. Cryan. New York, NY, Springer New York: 195-219.
-
Hu, P., Y. Lu, B.-X. Pan, and W.-H. Zhang (2022). "New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder." International Journal of Molecular Sciences 23(19): 11076.
-
Hu, S., Y. Chen, Y. Chen, and C. Wang (2021). "Depression and Anxiety Disorders in Patients With Inflammatory Bowel Disease." Front Psychiatry 12: 714057.
-
Huang, Y., J. Wu, H. Zhang, Y. Li, L. Wen, X. Tan, K. Cheng, Y. Liu, J. Pu, L. Liu, H. Wang, W. Li, S. W. Perry, M.-L. Wong, J. Licinio, P. Zheng, and P. Xie (2023). "The gut microbiome modulates the transformation of microglial subtypes." Molecular Psychiatry 28(4): 1611-1621.
-
Ikegami, M., H. Narabayashi, K. Nakata, M. Yamashita, Y. Sugi, Y. Fuji, H. Matsufuji, G. Harata, K. Yoda, and K. Miyazawa (2024). "Intervention in gut microbiota increases intestinal γ-aminobutyric acid and alleviates anxiety behavior: a possible mechanism via the action on intestinal epithelial cells." Frontiers in Cellular and Infection Microbiology 14: 1421791.
-
Inaba, T., K. Yamashiro, N. Kurita, Y. Ueno, N. Miyamoto, K. Hira, S. Nakajima, C. Kijima, R. Nakaguro, T. Urabe, and N. Hattori (2023). "Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion." CNS Neuroscience & Therapeutics 29(S1): 200-212.
-
Kelly, J. R., C. Minuto, J. F. Cryan, G. Clarke, and T. G. Dinan (2017). "Cross talk: the microbiota and neurodevelopmental disorders." Frontiers in Neuroscience 11: 490.
-
Kim, J. S. and C. B. de La Serre (2018). "Diet, gut microbiota composition and feeding behavior." Physiology & Behavior 192: 177-181.
-
Lamers, F., Y. Milaneschi, J. H. Smit, R. A. Schoevers, G. Wittenberg, and B. W. J. H. Penninx (2019). "Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety." Biological Psychiatry 85(10): 829-837.
-
Li, N., Q. Wang, Y. Wang, A. Sun, Y. Lin, Y. Jin, and X. Li (2019). "Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis." Stress 22(5): 592-602.
-
Lightman, S. L., M. T. Birnie, and B. L. Conway-Campbell (2020). "Dynamics of ACTH and Cortisol Secretion and Implications for Disease." Endocrine Reviews 41(3).
-
Lin, L., C. Li, Y. Zhang, L. Zhang, L. Gao, L. Jin, Y. Shu, and Y. Shen (2024). "Effects of an Akt-activating peptide obtained from walnut protein degradation on the prevention of memory impairment in mice." Food & Function 15(4): 2115-2130.
-
Liu, R. T., R. F. Walsh, and A. E. Sheehan (2019). "Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials." Neuroscience & Biobehavioral Reviews 102: 13-23.
-
Logsdon, A. F., M. A. Erickson, E. M. Rhea, T. S. Salameh, and W. A. Banks (2018). "Gut reactions: How the blood–brain barrier connects the microbiome and the brain." Experimental Biology and Medicine 243(2): 159-165.
-
Mac Giollabhui, N., C. Slaney, G. Hemani, É. M. Foley, P. J. van der Most, I. M. Nolte, H. Snieder, G. Davey Smith, G. M. Khandaker, and C. A. Hartman (2025). "Role of inflammation in depressive and anxiety disorders, affect, and cognition: genetic and non-genetic findings in the lifelines cohort study." Translational Psychiatry 15(1): 164.
-
Makris, A. P., M. Karianaki, K. I. Tsamis, and S. A. Paschou (2021). "The role of the gut-brain axis in depression: endocrine, neural, and immune pathways." Hormones 20(1): 1-12.
-
Mayer, E. A. (2011). "Gut feelings: the emerging biology of gut–brain communication." Nature Reviews Neuroscience 12(8): 453-466.
-
McGuinness, A. J., A. Loughman, J. A. Foster, and F. Jacka (2024). "Mood Disorders: The Gut Bacteriome and Beyond." Biological Psychiatry 95(4): 319-328.
-
Methiwala, H. N., B. Vaidya, V. K. Addanki, M. Bishnoi, S. S. Sharma, and K. K. Kondepudi (2021). "Gut microbiota in mental health and depression: role of pre/pro/synbiotics in their modulation." Food Funct 12(10): 4284-4314.
-
Min, X., G. Wang, Y. Cui, P. Meng, X. Hu, S. Liu, and Y. Wang (2023). "Association between inflammatory cytokines and symptoms of major depressive disorder in adults." Frontiers in Immunology Volume 14 - 2023.
-
NIMH. (2025). "National Institute of Mental Health, Major depression statistics." from https://www.nimh.nih.gov/health/statistics/major-depression.
-
O’Mahony, S. M., G. Clarke, Y. E. Borre, T. G. Dinan, and J. F. Cryan (2015). "Serotonin, tryptophan metabolism and the brain-gut-microbiome axis." Behavioural Brain Research 277: 32-48.
-
Patel, R. A., A. N. Panche, and S. N. Harke (2025). "Gut microbiome-gut brain axis-depression: interconnection." The World Journal of Biological Psychiatry 26(1): 1-36.
-
Peirce, J. M. and K. Alviña (2019). "The role of inflammation and the gut microbiome in depression and anxiety." Journal of Neuroscience Research 97(10): 1223-1241.
-
Raison, C. L., L. Capuron, and A. H. Miller (2006). "Cytokines sing the blues: inflammation and the pathogenesis of depression." Trends in Immunology 27(1): 24-31.
-
Rogers, G. B., D. J. Keating, R. L. Young, M. L. Wong, J. Licinio, and S. Wesselingh (2016). "From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways." Molecular Psychiatry 21(6): 738-748.
-
Rowland, I., G. Gibson, A. Heinken, K. Scott, J. Swann, I. Thiele, and K. Tuohy (2018). "Gut microbiota functions: metabolism of nutrients and other food components." European Journal of Nutrition 57(1): 1-24.
-
Shan, B., Z. Ai, S. Zeng, Y. Song, J. Song, Q. Zeng, Z. Liao, T. Wang, C. Huang, and D. Su (2020). "Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway." Psychoneuroendocrinology 117: 104699.
-
Silva, Y. P., A. Bernardi, and R. L. Frozza (2020). "The role of short-chain fatty acids from gut microbiota in gut-brain communication." Frontiers in Endocrinology 11: 508738.
-
Staudacher, H. M., C. J. Black, S. B. Teasdale, A. Mikocka-Walus, and L. Keefer (2023). "Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management." Nat Rev Gastroenterol Hepatol 20(9): 582-596.
-
Tung, T.-H., W.-D. Lai, H.-C. Lee, K.-P. Su, B. Panunggal, and S.-Y. Huang (2023). "Attenuation of Chronic Stress-Induced Depressive-like Symptoms by Fish Oil via Alleviating Neuroinflammation and Impaired Tryptophan Metabolism in Aging Rats." Journal of Agricultural and Food Chemistry 71(40): 14550-14561.
-
Virgintino, D., M. Errede, D. Robertson, C. Capobianco, F. Girolamo, A. Vimercati, M. Bertossi, and L. Roncali (2004). "Immunolocalization of tight junction proteins in the adult and developing human brain." Histochemistry and Cell Biology 122(1): 51-59.
-
Wang, X., R. Hu, F. Lin, T. Yang, Y. Lu, Z. Sun, T. Li, and J. Chen (2024). "Lactobacillus reuteri or Lactobacillus rhamnosus GG intervention facilitates gut barrier function, decreases corticosterone and ameliorates social behavior in LPS-exposed offspring." Food Research International 197: 115212.
-
WHO, W. H. O. (2023). "World Health Organization. “Depressive Disorder (Depression)." from https://www.who.int/news-room/fact-sheets/detail/depression.
-
Wong, M.-L., A. Inserra, M. Lewis, C. A. Mastronardi, L. Leong, J. Choo, S. Kentish, P. Xie, M. Morrison, and S. Wesselingh (2016). "Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition." Molecular Psychiatry 21(6): 797-805.
-
Xu, B., L.-M. Lang, S.-Z. Li, J.-R. Guo, J.-F. Wang, H.-M. Yang, and S. Lian (2019). "Microglia activated by excess cortisol induce HMGB1 acetylation and neuroinflammation in the hippocampal DG region of mice following cold exposure." Biomolecules 9(9): 426.
-
Xu, M., W. Li, X. Hu, and J. Zhang (2025). "Arecoline Alleviates Depression via Gut–Brain Axis Modulation, Neurotransmitter Balance, Neuroplasticity Enhancement, and Inflammation Reduction in CUMS Mice." Journal of Agricultural and Food Chemistry 73(17): 10201-10213.
-
Yang, H.-l., M.-M. Li, M.-F. Zhou, H.-S. Xu, F. Huan, N. Liu, R. Gao, J. Wang, N. Zhang, and L. Jiang (2021). "Links Between Gut Dysbiosis and Neurotransmitter Disturbance in Chronic Restraint Stress-Induced Depressive Behaviours: the Role of Inflammation." Inflammation 44(6): 2448-2462.
-
Yano, J. M., K. Yu, G. P. Donaldson, G. G. Shastri, P. Ann, L. Ma, C. R. Nagler, R. F. Ismagilov, S. K. Mazmanian, and E. Y. Hsiao (2015). "Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis." Cell 161(2): 264-276.
-
Yu, M., H. Jia, C. Zhou, Y. Yang, Y. Zhao, M. Yang, and Z. Zou (2017). "Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics." Journal of Pharmaceutical and Biomedical Analysis 138: 231-239.
-
Zhang, Q., Y. Bi, B. Zhang, Q. Jiang, C. K. Mou, L. Lei, Y. Deng, Y. Li, J. Yu, and W. Liu (2024). "Current landscape of fecal microbiota transplantation in treating depression." Frontiers in Immunology 15: 1416961.
-
Zhang, Y., P. Wang, C. Xia, Z. Wu, Z. Zhong, Y. Xu, Y. Zeng, H. Liu, R. Liu, and M. Liao (2020). "Fructooligosaccharides supplementation mitigated chronic stress-induced intestinal barrier impairment and neuroinflammation in mice." Journal of Functional Foods 72: 104060.
-
Zhao, H., X. Chen, L. Zhang, C. Tang, F. Meng, L. Zhou, P. Zhu, Z. Lu, and Y. Lu (2023). "Ingestion of Lacticaseibacillus Rhamnosus Fmb14 prevents depression-like behavior and brain neural activity via the microbiota–gut–brain axis in colitis mice." Food & Function 14(4): 1909-1928.
-
Zhou, L. and J. A. Foster (2015). "Psychobiotics and the gut–brain axis: in the pursuit of happiness." Neuropsychiatric Disease and Treatment: 715-723.
-
Zu, X., J. Xin, H. Xie, X. Xu, Y. Shen, J. Wang, S. Tian, Y. Wen, H. Li, J. Yang, and Y. Fang (2024). "Characteristics of gut microbiota and metabolic phenotype in patients with major depressive disorder based on multi-omics analysis." Journal of Affective Disorders 344: 563-576.
Related Content
Markers for Astrocyte Identification | Proteintech Group
How to culture mouse primary cerebral microvascular endothelial cells | Proteintech Group
Vascular dysfunction in Alzheimer’s Disease | Proteintech Group
How to Identify Activated Microglia | Proteintech Group
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

