Scientists Validate Biomarker Assay for Parkinson’s Disease!
A clinical study shows that α-synuclein seed amplification assays (SAAs) can reliably identify people with Parkinson’s disease.
Written by Jessica Lacoste, PhD, Postdoc at University of Toronto
Parkinson’s disease (PD) is a brain disorder that causes unintended or uncontrollable movements, such as shaking, stiffness, and difficulty with balance and coordination. PD is the second-most common neurodegenerative disorder, affecting 2-3% of the population over 65 years of age.
Mechanisms and pathophysiology
One of the defining pathological characteristics of PD is intracellular α-synuclein protein accumulation in neuronal cells (PMID: 28332488). The normal neuronal function of the α-synuclein protein is not fully understood, but it becomes neurotoxic when soluble α-synuclein monomers oligomerize and aggregate into Lewy bodies (PMID: 28332488).
Lewy body formation occurs by the following process:
-
α-synuclein monomers form oligomers
-
Oligomers combine to form small protofibrils
-
Protofibrils form large insoluble α-synuclein fibrils, which are the aggregates that make up Lewy bodies (PMID: 28332488)
The underlying triggers of accumulation and aggregation of α-synuclein are overproduction of the protein, genetic mutations that increase misfolding and oligomerization, or impaired degradation of native or misfolded α-synuclein (PMID: 28332488).
Biomarkers
Finding specific and sensitive biomarkers for PD has been at the forefront of research for decades. Accurate biomarkers can be used to assess disease risk, progression, or can enhance early diagnosis. Currently, the only widely accepted way to confirm a clinical PD diagnosis is by postmortem analysis of patient brain tissue. Validated biomarkers are now being studied in the clinic since they are a major need both in research and clinical care of patients with PD.
Recently, clinical studies showed that α-synuclein seed amplification assays (SAAs) on cerebrospinal fluid (CSF) samples are able to distinguish people with PD from healthy controls with high sensitivity and specificity (PMID: 34742348; PMID: 32342188).
Seed Amplification Assay (SAA)
Protein misfolding of α-synuclein in vitro follows a seeding nucleation mechanism, in which an aggregated α-synuclein protein (seed) can induce a conformational change in an identical native protein making it more aggregation prone (Figure 1) (PMID: 36653527). The aggregates can then assemble to form insoluble fibers (amyloid fibrils) that are resistant to degradation (PMID: 31427526).
In the SAA, recombinant α-synuclein (native protein) and CSF from patients (which may contain aggregated α-synuclein protein) are added into solution. Then, aggregation of α-synuclein is accelerated by cycles of fragmentation and elongation (Figure 1). Fragmentation increases the number of active seeds and elongation allows native (recombinant) protein to be converted into aggregates which then assemble into amyloid fibrils. This process of fragmentation and elongation is repeated in a cyclic manner to amplify the aggregated protein and thus the amyloid fibrils. The aggregates can then be detected by conventional methods, such as thioflavin T (ThT) fluorescence. ThT is a small molecule that gives strong fluorescence upon binding to amyloid fibrils (PMID: 28280572).
In this assay, there are three phases for formation of highly organized protein aggregates (Figure 1):
-
The lag or nucleation phase: During the lag phase, the early misfolding events leading to formation of seeds (aggregates) take place and therefore, there will be no observable aggregation.
-
The growth or elongation phase: Once enough seeds have been formed, there is a rapid increase in the formation of protofibrils and fibrils, resulting in the increased number of observable aggregates. If aggregated α-synuclein protein (seeds) are added in the reaction mixture (from the patient CSF), the lag phase is dramatically decreased because the reaction bypasses the first step where seeds must be formed. The aggregates are thus detected far quicker in samples where seeds were present.
-
The stationary phase: In the stationary phase, the substrate (recombinant α-synuclein) will have been used up, slowing down the aggregation. The reaction mixture then enters an equilibrium in which there is no further change in aggregation.
If aggregated α-synuclein protein (seeds) are added in the reaction mixture (from the patient CSF), the lag phase is dramatically decreased because the reaction bypasses the first step where seeds must be formed. The aggregates are thus detected far quicker in samples where seeds were present.
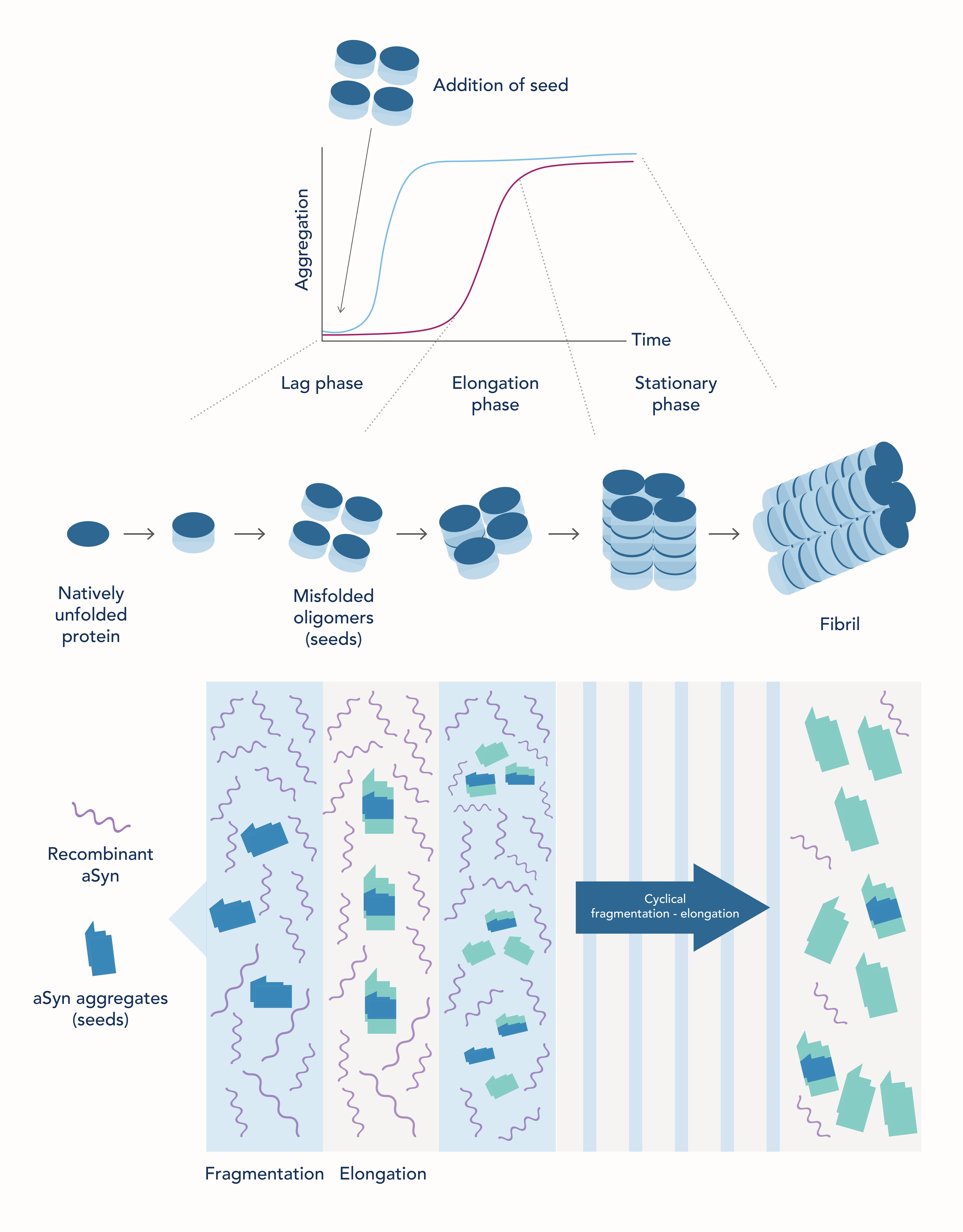
Figure 1. Schematic representation of the seeding/nucleation model of protein misfolding and aggregation (top) and of the SAA reaction (bottom). Adapted from Concha-Marambio et al., 2023 (PMID: 36653527).
A crucial role for α-synuclein SAA in Parkinson disease diagnostics
Previous clinical studies that assessed the performance of the α-synuclein SAA were done in a small sample size and so results were less conclusive. A recent study published in the Lancet (PMID: 37059509), extended the previous clinical studies on the use of α-synuclein SAA for in vivo molecular characterization of PD using a larger sample size in a more diverse range of PD patients. This study assessed the performance of α-synuclein SAAs in identifying heterogeneity among PD patients and used the data collected to identify early signs of PD pathophysiological changes in at-risk groups.
This study used the well characterized multicenter Parkinson's Progression Markers Initiative (PPMI) cohort which included people with PD with and without associated genetic variants, healthy controls, and people at risk for PD (either with prodromal features or non-manifesting carriers of genetic variants). There was a total of 1,123 participants enrolled in the study including 163 healthy controls and patients with PD or at-risk for PD.
The sensitivity of the assay was high, 87.7% of all PD patients tested positive in the α-synuclein-SAA. The specificity of the assay for healthy controls was 96.3%. This shows that the α-synuclein-SAA was highly accurate in differentiating PD patients from healthy controls. Among clinical features, hyposmia (decreased sense of smell) was the most robust predictor of a positive result by the α-synuclein-SAA, with a sensitivity of 97.2% for all participants with PD with hyposmia.
This study observed variability among genetic subgroups, especially among participants with the LRRK2 variant. The proportion of participants with positive α-synuclein SAA results was highest for PD patients with the GBA N409S genetic variant (95.9%), followed by sporadic PD (93.3%), and lowest for PD patients with the LRRK2 G2019S genetic variant (67.5%). Of the 310 non-manifesting carriers, only 25 participants (8%) tested positive (9% LRRK2 and 7% GBA).
What this means for the future: This advancement will help medical practitioners objectively define Parkinson’s Disease in patients. This validated assay will allow earlier diagnosis, targeted treatments, and more effective strategies for the prevention of disease.
Clinical studies use the terms sensitivity and specificity to assess the performance of biomarker assays. Sensitivity refers to the assay’s ability to designate an individual with disease as positive. The specificity of an assay is its ability to designate an individual who does not have a disease as negative.
References
1. PMID: 28332488. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017 Mar 23;3:17013. doi: 10.1038/nrdp.2017.13.
2. PMID: 34742348. Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM, Holguin B, Hughson AG, LaFontant DE, Caspell-Garcia C, Coffey CS, Mollon J, Hutten SJ, Merchant K, Heym RG, Soto C, Caughey B, Kang UJ. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson's disease. Acta Neuropathol Commun. 2021 Nov 6;9(1):179. doi: 10.1186/s40478-021-01282-8. Erratum in: Acta Neuropathol Commun. 2021 Nov 26;9(1):190.
3. PMID: 32342188. Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orrù CD, Antelmi E, Mammana A, Hughson AG, Calandra-Buonaura G, Ladogana A, Plazzi G, Cortelli P, Caughey B, Parchi P. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020 Jul;140(1):49-62. doi: 10.1007/s00401-020-02160-8. Epub 2020 Apr 27. Erratum in: Acta Neuropathol. 2020 Aug;140(2):245.
4. PMID: 36653527. Concha-Marambio L, Pritzkow S, Shahnawaz M, Farris CM, Soto C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat Protoc. 2023 Apr;18(4):1179-1196. doi: 10.1038/s41596-022-00787-3. Epub 2023 Jan 18.
5. PMID: 31427526. Araki K, Yagi N, Aoyama K, Choong CJ, Hayakawa H, Fujimura H, Nagai Y, Goto Y, Mochizuki H. Parkinson's disease is a type of amyloidosis featuring accumulation of amyloid fibrils of α-synuclein. Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17963-17969. doi: 10.1073/pnas.1906124116. Epub 2019 Aug 19.
6. PMID: 28280572. Xue C, Lin TY, Chang D, Guo Z. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R Soc Open Sci. 2017 Jan 4;4(1):160696. doi: 10.1098/rsos.160696.
7. PMID: 37059509. Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C; Parkinson's Progression Markers Initiative. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023 May;22(5):407-417. doi: 10.1016/S1474-4422(23)00109-6.
Related Content
Parkinson's disease pathway poster
Vascular dysfunction in Alzheimer's disease
Scientists discover Dynein-Driven motility in primary cilia
7 tips to successfully culture primary rodent neurons
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.