Nano Diamonds and Cancer
An introduction to nanoparticle delivery in cancer, written by guest blogger Veronika Benson.
So what exactly is so exceptional about ‘nano’ research?
Indeed, it is simply the small size; a nanoparticle is small enough to enter a live cell, but still has the potential to carry a substantial therapeutic payload or to instill luminescence center. In comparison to conventional macroscale materials, nanomaterials possess special optoelectronic, mechanic, and magnetic properties. Their relatively huge surface area provides a great amount of reactive groups that enable the binding of all different kinds of macromolecules. All these characteristics result in unique interactions with all types of biological entities such as: nucleic acids, proteins, membranes, organelles, or even whole cells. The type and output of such interactions then depend on the colloidal characteristic of each nanoparticle, the properties of the biological counterpart, and on thermodynamic exchanges between nanoparticle surfaces and a biological object. A great deal of scientific interest is focused on the use of nanoparticles as drug delivery agents. First, liposome and polymer nanoparticles have been researched, and after several decades, there are few clinical trials based on those materials ongoing, regarding cancer therapy. Especially in association with gene therapy, they are forming a completely new treatment approach. The first successful clinical trial in humans was published in 2010 in Nature (1). Here, Davis et al. used a cyclodextrin-based polymer nanoparticle decorated with polyethylene glycol-transferrin for tumor cell targeting and with siRNA specific to ribonucleotide reductase. The complexes (about 70nm in diameter) were administered intravenously in patients with metastatic melanoma. The therapeutic complex accumulated in tumors and resulted in decreased levels of the targeted mRNA and protein. It was the first report showing that the systemically administered siRNA delivered by a nanoparticle, triggered RNA interference in target tumor cells in humans.
Nanodiamonds in Cancer Research
Studies regarding the preparation and application of new diagnostic and therapeutic delivery systems are gaining more importance. New delivery systems are being sought out to identify a drug carrier with the ideal characteristics, such as efficient uptake at target sites or favorable pharmacokinetics, biodegradation, and system distribution. Recently, inorganic material has risen in popularity since its physiochemical properties can make it suitable for being used as diagnostic and therapeutic delivery vehicles. Nanodiamonds are a novel class of carbon material, which exhibits high compatibility with life systems, and possess other advantageous characteristics. Next to their excellent biocompatibility, they are quite versatile to bind and deliver different drugs, imaging agents, nucleic acids, and proteins. This material can also be readily scaled to commercial production (2). In contrast to an easy to see diamond decorating jewelry, the nanodiamonds – primarily of a particle size of approx. 5nm – can be produced through inexpensive large-scale synthesis based on the detonation of carbon-containing explosives. Ultrananocrystalline nanodiamond particles (1-150nm) are produced via a high-pressure high-temperature protocol. The type of production determines the size, shape, and surface properties of the nanodiamond which can be further modified allowing their specific use. In particular, nanodiamonds found their use in cancer therapy as drug delivery agents and sensors for real-time imaging. Up until now, there have been 84 publications regarding the use of nanodiamonds in cancer therapy and diagnosis. Most of the studies focus on the use of nanodiamonds in a combination with conventional drugs such as doxorubicin, paclitaxel, or cisplatin. The nanodiamonds are used as a colloid dispersion, or they are embedded in a polymer grid. Several studies describe that an additional coating of the nanodiamonds with a targeting molecule or cell penetrating peptide TAT brings a more effective anti-cancer effect. Later on, studies regarding nanodiamonds delivering nucleic acids emerged. Here, nucleic acids are mostly linked via a polymeric coating or an anchor.
The first evidence of the use of nanodiamonds in cancer therapy was published in 2010 by Guan et al. (3). The authors reported reduced proliferation of human cervical cancer cells (Hela cell line) as a result of the nanodiamond-mediated delivery of cisplatin and its release in an acidic intracellular environment. In 2011 Chow et al. (4) described the nanodiamond-mediated delivery of doxorubicin into treatment-resistant cancers such as mammary tumors and liver cancer. The paper also showed nanodiamonds enabled the delivery of the drug towards the tumor, but this approach also resulted in an increased treatment efficacy. Using doxorubicin-modified nanodiamonds overcame the drug efflux responsible for previous tumor resistance. The affected tumor cells underwent apoptosis, and tumor growth was inhibited significantly more than in the case of conventional doxorubicin-only treatment. Next to the increasing doxorubicin anticancer effect, the binding to nanodiamonds also reduced side toxicity effects in vivo.
 |
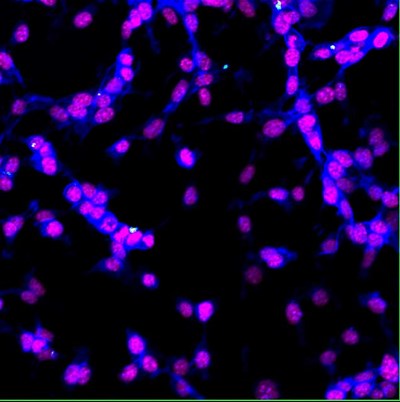 |
| Colon cancer cells (CT26.WT) internalize 35nm fluorescent nanodiamond (white). Cell nuclei stained with DAPI (magenta) and F-actin stained with Phalloidin (blue). Multicolor confocal analysis; Visualized by a confocal microscopy, Olympus FV1000, fixed cells, 40x, Eva Neuhӧferova, LaMBI. |
|
An optimal drug delivery system must exhibit minimal toxicity when applied without a therapeutic and is a limiting factor for a substantial amount of potential carriers. The reporting of Li et al. (5) describes the toxicity of nanodiamonds, taking effect in a serum-free environment. The serum proteins interact with the nanodiamonds and form a protein corona on the nanodiamond surface. The amount of serum proteins adsorbed on a nanodiamond surface was as high as 48 wt% at 37°C. When cells are exposed to nanodiamonds dispersed in a complete culture media with serum, the nanodiamonds exhibited no significant toxicity entering the intracellular compartment. However, after the internalization of the nanodiamonds dispersed in a serum-free media, the authors experienced abundant cell death. The adsorbed “protective” proteins stabilize the nanodiamond dispersion and reduce the size of aggregates. This is an important issue in using nanodiamonds for intravenous drug delivery.
A notable study reported by Xi et al. (6) discusses the use of nanodiamonds for the therapy of glioblastoma. Here, nanodiamonds serve as a delivery system for doxorubicin via a convection-enhanced delivery (CED). The CED approach is supposed to overcome the lack of penetration in a blood-brain barrier. This barrier represents the main obstacle in the treatment of glioblastoma because it prevents the sufficient accumulation of anticancer drugs in the brain after systemic administration.
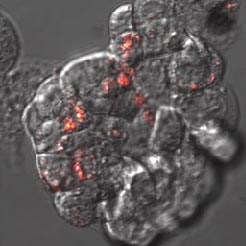 |
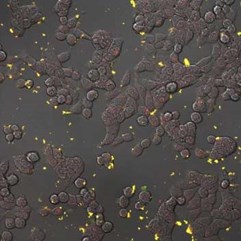 |
Fluorescent nanodiamonds perform as an internalization probe to monitor transfection of colon cancer cells HT-29. A) A pH sensitive sensor consisting of a nanodiamond (red) and fluorescein (green). After internalization, fluorescein signal diminishes but the signal of internalized fluorescent nanodiamonds remains visible. B) Detail of HT-29 cells with internalizing nanodiamonds (red). Visualized by a confocal microscopy, Olympus FV1000, live cells, 40x (B -zoom 4x), Veronika Benson, LaMBI.
Gene therapy holds great promise towards the personalization of cancer treatment. Here, an optimal delivery system is still missing, and nanomaterials are promising technological approach. Alhaddad et al. (7) reported the use of nanodiamonds for siRNA delivery into Ewing sarcoma cells. The in vitro study showed that complexes of nanodiamonds coated with a polymer and EWS-Fli1-specific siRNA triggered a reduction of the EWS-Fli1 gene as well as the protein expression. Next to delivery function, fluorescent nanodiamonds enable cargo tracking due to their intrinsic luminescence. Nanodiamond optical properties bring great opportunity to visualize the interactions with target cells and their compartments, as well as to monitor therapeutic load or cell movements which all lead to progress in biology and medicine.
So far, there are no clinical trials with nanodiamonds as a delivery system for an anti-cancer drug, but there are several patents. One is particularly interesting; this study reported the use of ND as a drug, specifically a drug generating free radicals for cancer treatment. Free radicals are produced on a nanodiamond’s surface after irradiation. A combination of nanodiamonds with a radio-sensitizing agent was even more effective (8).
In summary, the nanodiamond carrier performing a new, nano-specific interaction with target cells offers a new strategy of a drug delivery which is biocompatible and enhances treatment efficacy as well as treatment safety.
References
- Davis et al. Nature 2010; 464: 1067.
- Passari et al. Nanosci Nanotechnol 2015; 15(2):972.
- Guan et al. Small 2010; 6: 1514.
- Chow, et al., Sci Transl Med 2011; 3: 73ra21.
- Li et al. Biomaterials 2010; 31: 8410.
- Xi et al. Nanomedicine 2014; 10: 381.
- Alhaddad et al. PLoS One 2012; 7: e52207.
- Petit et al. 2014; FR 2993180 A1 20140117, WO 2014009930, A1 20140116.
Related Links
Nanodiamonds and Dental Fillings
About the Author
 |
Dr. Veronika Benson got her PhD in the field of biomedicine, and for the majority of her career she has been interested in the development of new approaches used in cancer diagnosis and therapy. Her projects have specialized on the fundamental research of cancer biology and immunology, specifically on drug-induced changes of signaling pathways and gene expression regulation. At present, she runs an interdisciplinary team within the Czech Academy of Sciences. Her Laboratory of Molecular Biology and Immunology (LaMBI) currently focuses on the development of nanodiamond systems, which will serve as a novel approach in the therapy of human pathologies such as cancer, diabetes, or neurodegenerative disorders. |
