Myc-Trap for both immunoprecipitation and affinity purification of Myc-tagged proteins
The Alpaca antibody advantage for pull-down of Myc-tagged proteins
Practical considerations and instructions
Introduction
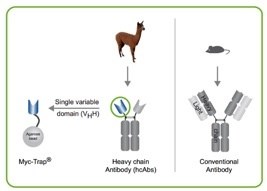
The ChromoTek Myc-Trap® is an affinity resin that consists of Myc-binding protein derived from an Alpaca single domain antibody, also called anti-Myc VHH or “anti-Myc nanobody”, which is coupled to agarose and magnetic agarose beads. The Myc-Trap provides advantages over conventional IgG antibodies when applied in immunoprecipitation (IP) and affinity purification experiments:
- no contamination of the protein of interest by heavy or light antibody chains (which may be the case when conventional antibodies are used as affinity resin)
- high specificity, sensitivity and affinity binding of the protein of interest enabling the pulldown or purification of low expressed/abundant proteins at high reproducibility
- gentle and effective elution to obtain native protein of interest
Optimize performance of your experiment
Myc-Trap’s performance has been optimized for immunoprecipitation (IP/Co-IP) and affinity purification of Myc-tagged proteins, which depends on whether you use a single Myc-tag, i.e. 1xMyc = EQKLISEEDL or a double Myc-tag fused to the protein of interest, i.e. 2xMyc = EQKLISEEDLEQKLISEEDL. Although both IP/Co-IP and protein purification can be done with all Myc-constructs, you can optimize your experimental performance by using either either a 1xMyc tagged or a 2xMyc-tagged protein. For an overview see table 1:
|
1xMyc-tagged protein |
2xMyc-tagged protein |
|
|
Table 1: Summary Myc-Trap as affinity resin for immunoprecipitation and affinity purification: Binding of 1xMyc and 2xMyc-fusion proteins results in application optimized performances. Note: Both IP/Co-IP and affinity purification can be done with all Myc constructs.
Description of binding and elution optimized performances
ChromoTek has investigated the binding mechanism of Myc-Trap in order to validate the function of this affinity resin. As a result, 2xMyc-tagged proteins are tighter bound to Myc-Trap than Myc-fusion proteins, which is manifested in different dissociation constants KD. These result from different binding mechanisms (for an introduction and discussion of the Myc-Trap’s epitope, see whitepaper How to plan an IP of your Myc-tagged protein when using the ChromoTek Myc-Trap).
Kinetic paramters are shown for 1xMyc-Maltose Binding Protein (MBP) and 2xMyc-MBP in table 2. Myc-Trap binds 1xMyc-tagged MBP tightly with a low KD of 400nM. This KD value already matches general requirements of immunoprecipitation. However, 2xMyc-tagged MBP is about 1,000 times stronger bound with a KD of just 0.5nM. These kinetic values explain the high pulldown and binding effectiveness of 2xMyc-tagged protein and the gentle elution capability of Myc-tagged protein when using Myc-Trap.
Because of the fast binding of 1xMyc- and 2xMyc-fusion proteins to the affinity resin Myc-Trap (high binding rates kon), the binding of Myc-fusions is already completed within 30 minutes. However, the low dissociation rate (koff) of bound 2xMyc-fusion proteins allows for prolonged incubation and washing, whereas the relative high dissociation rate (koff) of Myc-fusion protein requires instant washing with short durations.
|
Kinetic parameter |
1xMyc-MBP |
2xMyc-MBP |
|
Dissociation constant KD |
400nM |
0.5nM |
|
Binding rates kon |
5.3 x 105 M-1 s-1 |
2.9 x 105 M-1 s-1 |
|
Dissociation rate koff |
2.1 x 10-1 s-1 |
1.4 x 10-4 s-1 |
Table 2: Kinetic parameters of 1xMyc and 2xMyc-fusion proteins bound to Myc-Trap Experiments were conducted using Maltose Binding Protein (MBP) fused to Myc-tag. The KD value for binding 1xMyc-MBP indicates strong binding and is in range what is generally required for IP. It does allow gentle and effective elution. The KD value for 2xMyc-MBP is about 1,000 fold smaller, indicating a considerable increase of affinity and binding strength and demonstrating the outstanding IP performance of Myc-Trap. It does enable binding of 2xMyc-tagged proteins at low concentrations and under harsh binding conditions.
There are various elution options dependent on your experimental strategy, i.e. whether you require effective/gentle elution or effective pull-down/binding. Four elution options and their performances are presented in table 3. As you can see, the effectiveness of these options actually depends on whether you immunoprecipitate/purify 1xMyc- or 2xMyc-tagged protein.
- If you like to effectively or gently elute your protein of interest in native conditions use 1xMyc-tagged protein in your experiement
- If you like to effectively pull-down/bind low expressed/abundant proteins, or low concentration proteins use 2xMyc-tagged protein in your experiment
|
Elution with |
Elution Effectiveness |
|
|
1xMyc-MBP |
2xMyc-MBP |
|
|
+++ |
+ |
|
|
Urea (8 M) |
+++ |
+ |
|
Glycine (0.2 M) pH 2.5 |
+ |
+ |
Table 3: Effectiveness of Myc-Trap elution options. Experiments were conducted using 1x and 2xMyc-tagged Maltose Binding Protein (MBP). Instead of 2x Myc-tag also 3xMyc-tagged protein can be used. Abbreviations:
o: <50%, +: ca. 50%, ++: ca.70%, +++ ca. 90% of bound protein eluted.
Afore data shall enable you to consider the optimal conditions for your experiment with Myc-Trap. Nevertheless, a summary of experimental design considerations is presented in table 4. If you like to obtain more detailed information on discussed matter or information about the Myc-Trap’s epitope and how Myc-Trap binds 1xMyc- and 2xMyc-fusion proteins please refer to our whitepaper How to plan an IP of your Myc-tagged protein when using the ChromoTek Myc-Trap.
|
1xMyc-tagged protein |
2xMyc-tagged protein |
|
|
Table 4: Summary of experimental design considerations
For more information
