International Unit-based Specific Activity Determination for HumanKine® Products
Recombinant protein biological activity can be quantified by international units (IU) based on comparisons to a centralized set of standards
What are International Units?
International Units (IU) are a measure of the biological effect of a substance. The IU is commonly used to quantify biologically active substances such as recombinant proteins, vitamins, hormones, medications, and vaccines. With regard to the recombinant protein field, IUs have become a particularly important way to compare activity between different commercially available forms of the same protein as the IU itself can serve as a common unit of activity quantification. Most determinations of IU are performed using a centralized set of WHO international standards or reference reagents. These materials have been assigned an agreed-upon potency based on an international collaborative study and can in turn be used as a reference material from which the potency of other products can be determined.
IU Determination Procedure
The specific activity of a recombinant protein in IU is typically determined by running the protein against its applicable WHO standard on the same assay and comparing the ratio of activity between the two products. As the WHO standard has an assigned IU potency, the potency of the recombinant protein can be determined based on the ratio of the assay readout (ie: ng/ml, ug/mL, etc.). The specific activity of HumanKine® products is determined using the following four steps:
- Determine specific activity/potency of standard material
- Run both standard and HumanKine® products on the same assay
- Compare assay readouts (EC50s) to each other. Specific activity is determined by the ratio of standard to HumanKine®
- Based on the ratio, a specific activity in IU can be assigned to the HumanKine® product
Sample IU Calculation: IL-7 (HZ-1281)
Below is a step-by-step example of how the specific activity of HumanKine®’s IL-7 protein was determined:
1. Determine specific activity of standard material
-
- Standard used: WHO Reference Reagent IL-7 (NIBSC cat no: 90/530)
- Assigned potency of standard: 100,000 units/ampoule
- Amount (mass) of IL-7 in ampoule: 1 ug
- Specific activity of standard: 100,000 U/ug
2. Run standard and HumanKine® product on same assay
- Both the HumanKine® and WHO products were run on the same assay testing their ability to induce the proliferation of mouse 2E8 cells in a dose-dependent manner. From this assay an EC50 in ng/mL for both products was determined.
HumanKine® Activity Curve
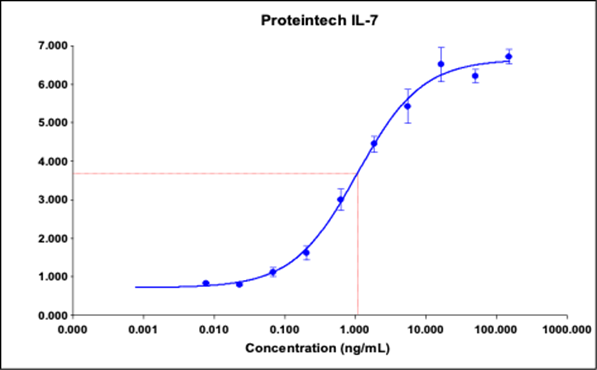
HumanKine® EC50: 1.079 ng/mL
WHO (NIBSC) Standard Activity Curve
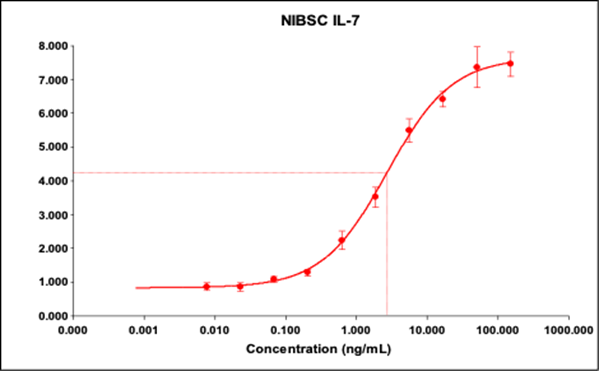
WHO Standard EC50: 2.727 ng/mL
3. Compare EC50s: specific activity is determined by the ratio of standard to HumanKine® activity
|
WHO Standard EC50 |
HumanKine® EC50 |
Ratio |
|
2.727 ng/mL |
1.079 ng/mL |
2.53 |
4. Based on ratio, assign specific activity to HumanKine® product
HumanKine® specific activity = Standard specific activity x Ratio
|
WHO Standard Specific Activity |
Ratio |
HumanKine® Specific Activity |
|
1x108 IU/mg |
2.53 |
2.53x108 IU/mg |
Additional Considerations for Specific Activity Determination
The most accurate method of determining the specific activity of a product is through direct comparison and calibration with the standard. Methods suggesting that a protein's activity can be formulaically converted from ng/mL to IU/mg by assuming that the amount of IU/mg is consistent between proteins should be avoided as the assigned potency of an international standard varies greatly for each protein. Additionally, the determination of the protein’s bioactivity is highly dependent upon the parameters of the assay method itself. Many factors can affect assay sensitivity, including cell responsiveness, media formulation, duration of stimulation, etc. As such, despite using the same standards for calibration, differences in assay methods may result in different specific activities being reported for the same protein by different manufacturers.
|
Protein |
NIBSC Standard |
Proteintech |
Competitor 1 |
Competitor 2 |
Competitor 3 |
|
IL-7 |
90/530 |
2.53x108 IU/mg Assay Method: Proliferation of mouse 2E8 cells |
1x108 IU/mg Assay Method: Proliferation of PHA-activated human peripheral blood lymphocytes |
1.17x108 IU/mg Assay Method: Proliferation of mouse 2E8 cells |
5x107 IU/mg Assay Method: Proliferation of mouse IxN/2B cells |
It is always best to test out a protein at different dilutions to determine which concentration works best for your desired application. Specific activity values provided on the Proteintech website are for reference use only and are based on historical testing against WHO standards. However, this method of specific activity determination is not routinely tested for HumanKine® products.
Related Content
HumanKine Cytokines & Growth Factors
How to choose your recombinant proteins, cytokines & growth factors
What do you get when you buy a GMP-grade product?

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.