Immunoprecipitation of Myc-tagged proteins – How it works
ChromoTek Myc-Trap® is an optimized tool for immunoprecipitation (IP/Co-IP)
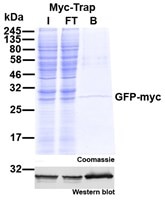
ChromoTek Myc-Trap® is an effective optimized tool for immunoprecipitation (IP/Co-IP) and affinity purification of Myc-tagged proteins. Depending on whether you use a single Myc-tag, i.e. 1xMyc (EQKLISEEDL) or a double Myc-tag fused to the protein of interest, i.e. 2xMyc (EQKLISEEDLEQKLISEEDL) you can adjust your experiment. Here, we introduce the epitope of Myc-Trap to discuss its performance for the aforementioned applications.
Now, should I use 1xMyc-tag or 2xMyc-tag?
Both IP/Co-IP and protein purification can be done with all Myc constructs. However, if you want to optimize your experimental performance, you may choose either a 1xMyc tagged or a 2xMyc-tagged protein:
1xMyc-tagged protein for effective elution:
- Gentle elution of native fusion protein
- One step elution
- Small volume to concentrate fusion protein
2xMyc-tagged protein for effective binding:
- Low expressed/concentration proteins
- Complete binding
- Extraordinary high binding affinity
- Stringent wash conditions for high pure results
Instead of 2xMyc-tag also 3xMyc-tag can be used.
The performance differences result from the Myc-Trap epitope and binding
The Myc-Trap was designed to recognize the c-Myc peptide EQKLISEEDL. We have determined the resulting epitope of the Myc-tag-binder using a peptide micro-array and an alanine scan of the c-Myc peptide. As a result, we found that the minimal epitope of the ChromoTek Myc-tag-binder is the sequence LISEEDL. Within this motif, the amino acid residues isoleucine (I), aspartic acid (D) and the last leucine (L) are essential for binding:
EQKLISEEDL
The highest binding, however, is accomplished when using a 2xMyc peptide (EQKLISEEDLEQKLISEEDL). It is likely that the ChromoTek Myc-tag binder interacts strongly with the second LISEEDL motif and then additionally with the first EQKLI motif:
EQKLISEEDLEQKLISEEDL
The sum of these two binding events yields a high-affinity interaction with the 2xMyc peptide, which is expressed in a significantly smaller dissociation constant than the interaction of Myc-fusion protein to the Myc-Trap. In addition, this explains why bound 1xMyc-fusion protein is eluted more gently from Myc-Trap than 2xMyc-tagged fusion protein. A detailed discussion on kinetic aspects and resulting experimental design options can be found here:
The Myc-Trap does NOT bind endogenous c-Myc protein
The Myc-tag is derived from the c-Myc transcription factor. This protein is engaged in cell cycle progression, apoptosis, and cellular transformation. Thus, one could expect binding of endogenous c-Myc protein. However, the epitope data described above may explain why the ChromoTek Myc-Trap does not bind any c-Myc protein. Some epitope residues that we have shown to be crucial for binding are actually buried in the three-dimensional structure of the c-Myc protein. Hence, under native conditions, c-Myc protein is not a suitable binding partner for the ChromoTek Myc-trap.
You want to test the Myc-Trap for yourself?
Myc-Trap Technology
The ChromoTek Myc-Trap is an anti-Myc single domain antibody from Alpaca, also called VHH or “nanobody”, coupled to a matrix like agarose beads. This affinity resin provides some advantages over conventional IgG antibodies when applied in immunoprecipitation (IP) and affinity purification experiments:
- no contamination of the protein of interest by heavy or light antibody chains (which may be the case when conventional antibodies are used as affinity resin)
- high specificity, sensitivity and affinity binding of the protein of interest enabling the pulldown or affinity purification of low expressed proteins with high reproducibility
There is one more thing: ChromoTek's anti-Myc-tag antibody 9E1 (rat monoclonal) is performing significantly better in Western Blot than the widespread 9E10 antibody.
