Immune Checkpoint Inhibitor Therapy: Revolutionizing Cancer Treatment
Written by Sepideh Jahangiri, PhD Candidate at the University of Montreal
In the late 19th century, William B. Coley laid the groundwork for modern immunotherapy. As an orthopedic surgeon, Coley observed that some of his cancer patients with post-surgical infections experienced spontaneous tumor regression. He hypothesized that the immune system, triggered by infections, could play a role in fighting cancer. In 1891, he began treating cancer patients by injecting them with mixtures of live and inactivated bacteria, including Streptococcus pyogenes and Serratia marcescens, in an effort to induce an immune response. His concoction, known as "Coley's toxin," achieved remarkable remissions in several cancer types, but the lack of a clear mechanism of action, coupled with the risks of infection, led to skepticism in the medical community (1).
It wasn’t until decades later that researchers understood the immunological principles behind Coley’s work. Skepticism about the immune system's role in combating cancer was widespread. For instance, a widely referenced study in the British Journal of Cancer found no evidence of immune response to 27 spontaneous tumors in mice, concluding that immune reactions in transplanted tumor models were more likely artifacts of viral or chemical induction (2). Another commentary on the challenges of cancer immunotherapy at the time expressed doubt about its feasibility, stating it would be "as difficult to reject the right ear and leave the left ear intact as it is to immunize against cancer" (3). Despite early setbacks due to significant toxicities and unpredictable responses, these efforts paved the way for the more refined and effective immune-based cancer therapies we see today.
In 2013, Science magazine named cancer immunotherapy the "Breakthrough of the Year," surpassing other advancements and signaling a pivotal moment in oncology. This recognition was driven by clinical trials demonstrating that immunotherapies could effectively treat advanced and metastatic cancers, significantly extending patient survival. The progress in immunotherapy has been marked by groundbreaking discoveries by James Allison, director of CRI’s Scientific Advisory Council, and Tasuku Honjo, Director of Center for Cancer Immunotherapy and Immunobiology (CCII), whose work on immune checkpoint proteins—programmed cell death-1 (PD-1), its ligand PD-L1, and cytotoxic T lymphocyte-associated protein 4 (CTLA-4)—earned them the 2018 Nobel Prize in Physiology or Medicine (4).
Immunoediting: The Evolving Battle Between Cancer and the Immune System
From an immunological perspective, the concept of immunoediting explains how cancer evolves in response to the immune system. The immune system is constantly engaged in a delicate balancing act, recognizing and eliminating abnormal cells while maintaining tolerance to normal tissue. Immunoediting consists of three phases: elimination, equilibrium, and escape.
- In the elimination phase, the immune system detects and destroys cancer cells before they become clinically significant. Immune cells like cytotoxic T cells, natural killer (NK) cells, and macrophages recognize tumor antigens and eliminate the malignant cells.
- In the equilibrium phase, some cancer cells survive by mutating and becoming less visible to the immune system. During this phase, cancer is kept in check but not entirely eradicated.
- In the escape phase, cancer cells evade immune detection altogether, either by downregulating MHC class I molecules to mask tumor antigens or by creating an immunosuppressive tumor microenvironment (TME). The TME becomes rich with molecules like PD-1, PD-L1, and CTLA-4, which inhibit immune cell activation and promote tumor growth.
Immunotherapy, particularly through immune checkpoint inhibitors (ICIs), seeks to reverse this immune escape. By targeting the brakes on the immune system (such as PD-1 and CTLA-4), ICIs reinvigorate immune cells to recognize and attack tumor cells, even in the hostile environment of the TME (5).
Mechanisms of Immune Checkpoints: PD-1 and CTLA-4
The immune system must strike a fine balance between attacking pathogens and maintaining tolerance to prevent autoimmunity. PD-1 and CTLA-4 are two of the most important co-inhibitory receptors that help regulate this balance. While both receptors function to suppress T-cell activity, their mechanisms of action and locations of expression differ.
- CTLA-4 primarily acts in the early stages of T-cell activation in lymphoid tissues. When naïve T cells encounter antigen-presenting cells, CTLA-4 competes with the costimulatory receptor CD28 for binding to the B7 molecules on these antigen-presenting cells. By doing so, CTLA-4 dampens the initial activation of T cells, acting as a brake to prevent uncontrolled immune activation (6).
- PD-1, on the other hand, operates at later stages of the immune response, predominantly in peripheral tissues where immune cells engage in effector functions. PD-1 is expressed on activated T cells, B cells, and myeloid cells, and when it binds to its ligands (PD-L1 or PD-L2), it sends an inhibitory signal to T cells, reducing their activity. This mechanism is crucial for maintaining immune homeostasis and preventing autoimmunity in healthy tissues (7).
By inhibiting these checkpoints, immune checkpoint inhibitors unleash T cells to perform their tumor-killing functions. Blocking PD-1 or CTLA-4 pathways leads to a more sustained immune response, allowing the immune system to break through the defenses that tumors use to evade detection. This has resulted in unprecedented clinical responses in a variety of cancers, particularly in tumors that were previously considered difficult or impossible to treat.
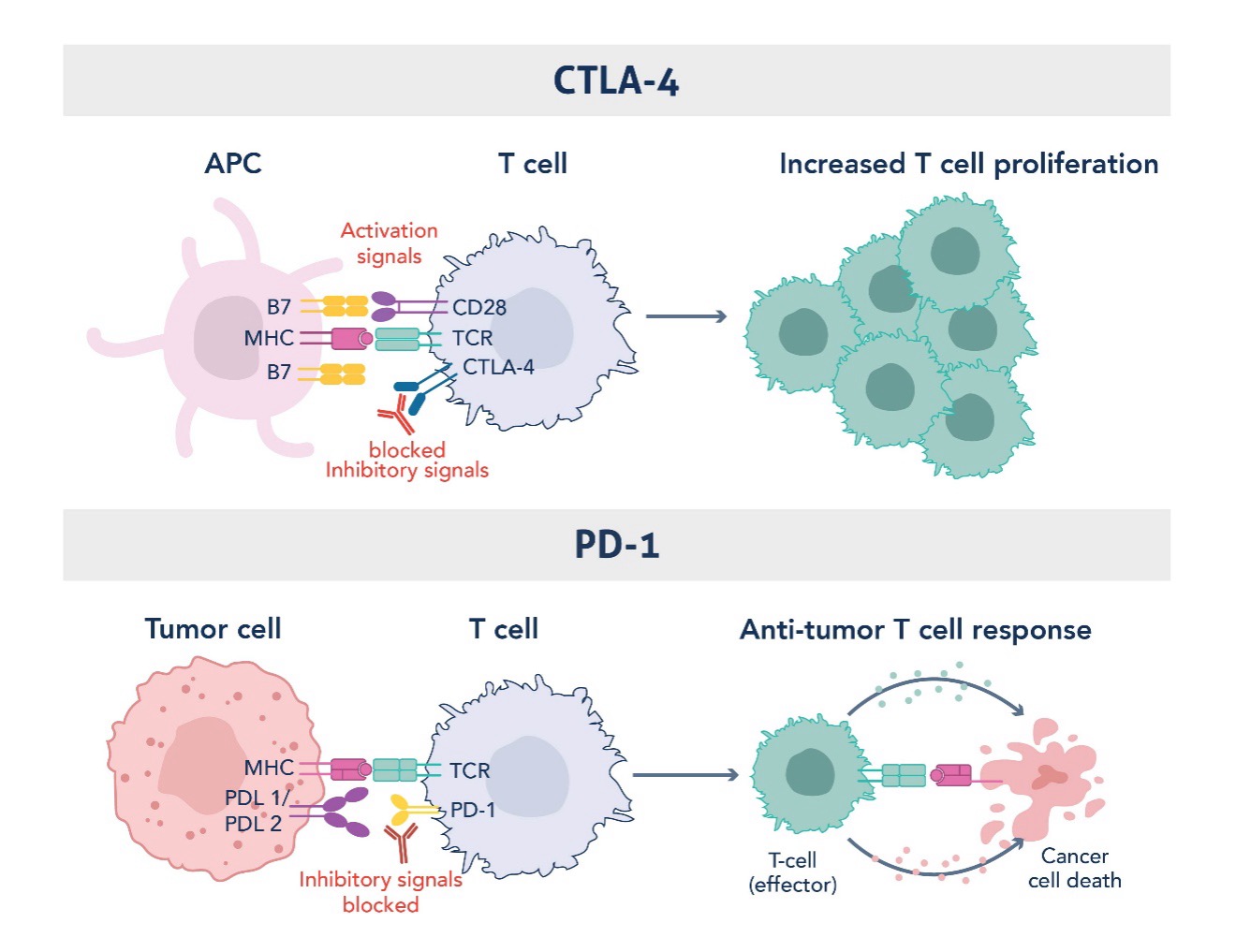
Figure 1. Mechanism of Action of CTLA-4 and PD1 Immune Checkpoints in Restraining Uncontrolled T Cell Activation and Function. Graphic adapted from Sharma et al., 2023 (PMID: 37059068).
Landmark Therapies and Expanding Horizons
The FDA first approved ipilimumab, a CTLA-4 inhibitor, in 2011 for the treatment of advanced melanoma. This was followed by the approval of pembrolizumab, an anti-PD-1 therapy, in 2014 for refractory melanoma (8). Pembrolizumab has since become a first-line treatment for various malignancies, including non-small cell lung cancer (NSCLC), metastatic triple-negative breast cancer, advanced urothelial carcinoma, and cervical cancer.
Researchers continue to explore new checkpoint pathways, such as LAG-3, TIGIT, VISTA, B7-H3, IDO, TIM-3, CD47, CD39, CD73, CD40, OX40, TIM3, and GITR, which hold the potential to further improve response rates and overcome resistance. These advancements aim to expand the horizons of ICI therapy, improving efficacy across various cancer types by targeting additional, non-redundant pathways. Additionally, the combination of ICIs with other treatments, such as chemotherapy, radiation, or targeted therapies—including but not limited to focused ultrasound, photodynamic therapy, nanoparticle delivery techniques, and oncolytic viruses—is being investigated to improve efficacy, overcome therapeutic resistance, and minimize adverse effects. These strategies aim to provide more durable responses and better outcomes for a broader range of cancers.
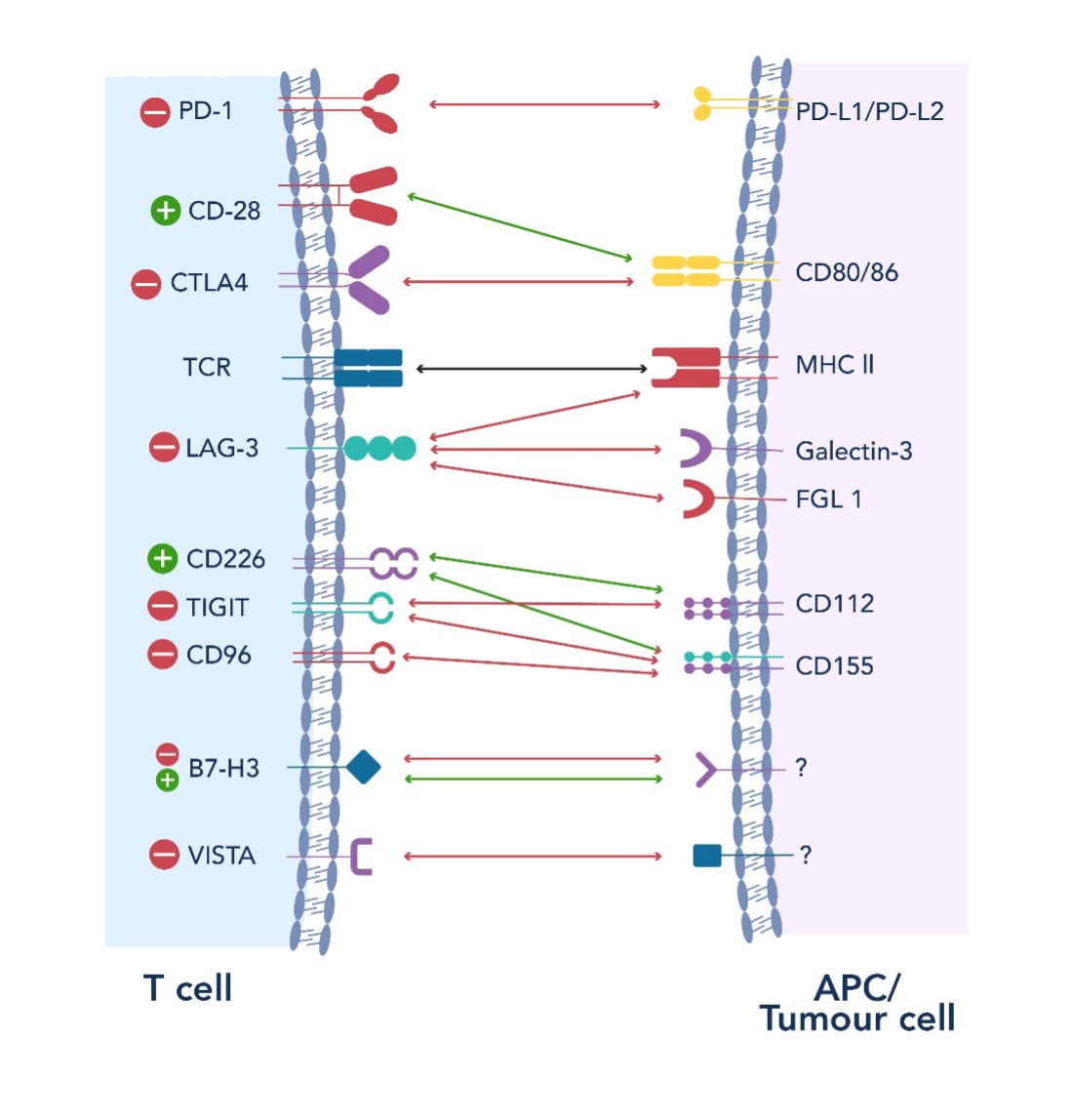
Figure 2. Overview of the Novel Immune Checkpoint Pathways in Cancer Progression and Therapy
Future Directions in Personalized Immunotherapy
The future directions of personalized immunotherapy in the realm of ICI therapy are poised for significant advancement. As the field evolves, there is a pressing need to deepen our understanding of the mechanisms behind checkpoint molecules and develop new combinatorial therapies targeting diverse kinds of checkpoint inhibitors. Innovative next-generation antibodies such as bispecific antibodies and antibody-drug conjugates aimed at achieving more effective antitumor responses are also currently under investigation. Personalized approaches, including neoantigen vaccines, expanded tumor-infiltrating lymphocytes (TILs), and biomarker-driven therapies, are designed to optimize treatment outcomes while minimizing side effects. Moreover, personalized multi-omics and comprehensive immune profiling hold the potential to identify tumor signatures prior to therapy, allowing for tailored treatment strategies. Notably, the application of ICI in neoadjuvant settings has demonstrated significant tumor shrinkage and improved immune responses, as evidenced by studies showing major pathological responses in a high percentage of patients with locally advanced dMMR colon and rectal cancers (9, 10). These studies not only underscore the potential of ICI to supplant traditional chemotherapy but also highlight the need for larger studies with longer follow-up to validate these findings. To optimize the current and future clinical use of ICI therapies, it is essential to define eligibility by determining the right timing, combination, sequence, and delivery of cancer immunotherapy (11). Accurate biomarkers must be identified to predict efficacy and adverse effects (12), with an emphasis on prompt diagnosis and management of any adverse events. Continued preclinical and clinical research is essential to advance our understanding of immune checkpoints and to refine management guidelines, ultimately paving the way for more effective and personalized treatment options in cancer immunotherapy.
References:
- Carswell E, Old LJ, Kassel R, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proceedings of the National Academy of Sciences. 1975;72(9):3666-70.
- Hewitt H, Blake E, Walder A. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. British Journal of Cancer. 1976;33(3):241-59.
- Woglom W. Immunity to Transplantable Tumours. 1929.
- Jennifer C-F. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432-3.
- Gubin MM, Vesely MD. Cancer Immunoediting in the Era of Immuno-oncology. Clinical Cancer Research. 2022;28(18):3917-28.
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discovery. 2018;8(9):1069-86.
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nature Reviews Immunology. 2018;18(3):153-67.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369(2):122-33.
- Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. New England Journal of Medicine. 2024;390(21):1949-58.
- Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. New England Journal of Medicine. 2022;386(25):2363-76.
- Kwon M, Jung H, Nam G-H, Kim I-S. The right Timing, right combination, right sequence, and right delivery for Cancer immunotherapy. Journal of Controlled Release. 2021;331:321-34.
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews Cancer. 2019;19(3):133-50.

Related Content
Understanding Liver Cancer: Causes, diagnostics, and therapeutic avenues
m5C Modifications in RNA and Cancer
The Role of Autophagy in Cancer
Cancer stem cells as a key to cure cancer
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
