How to culture Bone Marrow Derived Macrophages
Our top tips to successfully extract and culture murine Bone Marrow Derived Macrophages
Macrophages are key cells of the adaptive immune system and are implicated in many diseases. They can be studied in vitro using macrophage-like cell lines such as the murine RAW264.7 line. However, cell lines are immortalized and often do not reflect the canonical function of the cells. Primary macrophages can be extracted and cultured from murine bone marrow and differentiated into Bone Marrow Derived Macrophages (BMDMs). BMDMs represent a more accurate model for macrophage function. Since primary cells can be tricky to work with, we have collated some tips for your experiment.
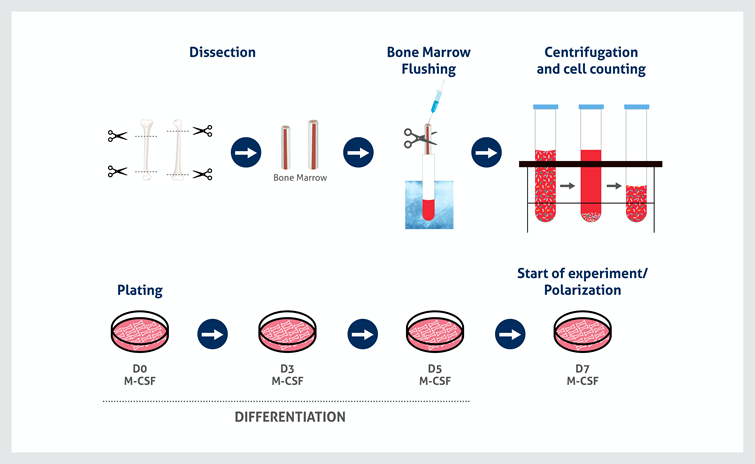
Figure 1: Workflow for the extraction and culture of murine BMDMs.
Here are our top tips for the successful extraction and culture of BMDMs:
- Keep it clean! Remove all tissue and clean the bones thoroughly with 70% ethanol before cutting the edges of the bones to avoid contamination of the bone marrow. The outside of the bones will not be sterile and this could contaminate the downstream culture. It is also recommended to supplement bone marrow culture medium with penicillin/streptomycin to further minimize contamination risk. If possible, carry out the extraction in a sterile environment. Cells present on the outside of the bones could end up in the bone marrow extract and outgrow the macrophage precursors.
- Create a single cell suspension. Dissociate cell clumps using a needle and syringe. The marrow might be flushed out in large cell clumps, but these can be broken up by flushing up and down with a needle to create a single cell suspension. Once the clumps have been removed, the cell suspension should also be filtered with a 70uM cell strainer to remove the bone debris.
- Lyse red blood cells from the bone marrow. This will concentrate your sample for macrophage precursors and increase your BMDM yield.
- Cell density matters. Count and check the viability of your bone marrow cells using a traditional hemacytometer and a microscope before plating for differentiation. Bone marrow cells are very small and automated cell counters might not count cell concentration accurately. BM cells are sensitive to their cell density: aim for 1x106 cells/mL.
- Use the right plastic. Differentiate your macrophages on non-tissue culture coated plastic (sterile bacterial dishes). They will otherwise become too adherent and you may be unable to detach them to re-seed for your experiments.
- Stick to stringent cell feeding. BMDM medium must contain Macrophage Colony Stimulating Factor (M-CSF) – this can use either the recombinant form of the protein (10ng/mL) or conditioned medium from murine fibroblast cells L929 that naturally secrete it (20% v/v with your medium). On day 4 post-extraction, add an equal volume of fresh medium to your culture, thus doubling the medium volume. Add this drop-wise so as not to disturb the cells. By day 7 post-extraction, the macrophages will be differentiated and can now be detached and re-seeded for the experiment. 50% of the medium must be refreshed every 48h thereafter to maintain the cell viability. Do not fully replace the culture medium as primary cells are sensitive to their secreted autocrine factors.
- Do not disturb the early culture. Although it can be tempting to check on the cells in the days immediately after extraction, do not touch the culture dishes for the first 4 days to allow the cells to attach.
- Timing is important. The BMDMs will be differentiated 7 days post-extraction. Experiments (including macrophage polarization if needed) should be started at that point and last no longer than a week. After this, the cells may no longer be representative of the macrophage phenotype and genotype.
Markers to study BMDMs:
|
|
||
|
|
Further reading:
Rios, F. J., Touyz, R. M., & Montezano, A. C. (2017). Isolation and differentiation of murine macrophages. In Hypertension (pp. 297-309). Humana Press, New York, NY.
Manzanero, S. (2012). Generation of mouse bone marrow-derived macrophages. In Leucocytes (pp. 177-181). Humana Press.
Assouvie, A., Daley-Bauer, L. P., & Rousselet, G. (2018). Growing murine bone marrow-derived macrophages. In Macrophages (pp. 29-33). Humana Press, New York, NY.
Blog written by Lucie Reboud, Intern at Proteintech, PhD student in cancer research at the University of Manchester.
Related Content
7 Tips for successfully culture primary rodent neurons

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
