Tips and tricks for immunoprecipitation of low abundant proteins
For a successful IP, the abundance or expression level of the POI is crucial. If a POI is strongly expressed and highly abundant, then the IP will most likely be straightforward, with no difficulties.
Protein abundance is crucial for immunoprecipitation
During an immunoprecipitation (IP), the protein of interest (POI) is pulled down with a specific antibody or Nanobody conjugated to beads such as agarose or magnetic agarose. Unbound sample content, for example, other proteins, cell debris, and lipids, is removed by washing, while the precipitated protein is enriched on the beads. For a successful IP, the abundance or expression level of the POI is crucial; unfortunately, this is frequently forgotten. If a POI is strongly expressed and highly abundant, then the IP will most likely be straightforward, with no difficulties. However, if the POI is present at a low abundance or expression level, optimization of the IP protocol may be necessary.
Below are some tips and tricks for how to successfully immunoprecipitate a low abundant POI.
Use an antibody or Nanobody with high affinity
Use a high affinity, low dissociation constant KD Nanobody for effective pulldown of small amounts of POI. This is because, according to steady state binding equations, only 50% of a POI is bound when its concentration equals the KD. Therefore, ideally, the POI should be present at a concentration that exceeds the KD of the antibody/Nanobody used for IP, to bind as much POI as possible and keep the amount of unbound POI as low as possible. If the antibody’s affinity is too low and its dissociation constant is too high respectively, the POI, although present in the cell lysate, may not be precipitated, which can lead to a false negative IP result.
Fortunately, for many Nanobodies, high affinity and high specificity go hand in hand and ensure a low background of cellular proteins, which is a prerequisite for the IP of low abundant proteins.
ChromoTek’s GFP-Trap® is an IP resin for immunoprecipitation of GFP fusion proteins. It comprises an anti-GFP Nanobody conjugated to agarose, magnetic agarose, magnetic particles M-270, and 96-well plates. GFP-Trap binds GFP and its commonly used variants with an extraordinarily high affinity with a dissociation constant of 1 pM. It can effectively precipitate even the lowest amounts or smallest concentrations of GFP-tagged proteins to the detection level as shown in the figure below. Here, EGFP at 4 concentrations, which correspond to abundances considered very high, high, low, and very low, have been immunoprecipitated and analyzed by SDS-PAGE and Western blotting. At very high and high EGFP abundances, the EGFP band of the bound fractions is detected on SDS-PAGE. At low and very low abundances, EGFP is no longer detectable on SDS-PAGE, although the EGFP band of the bound fractions is detected in Western blotting experiments. Even at the concentration considered very low abundance, the IP performance of the GFP-Trap is so good that no EGFP can be detected in the flow-through by Western blotting.
Immunoprecipitation of EGFP with GFP-Trap Agarose
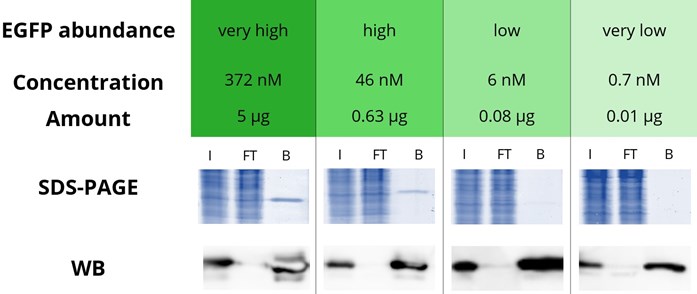
4 defined amounts of EGFP were spiked into 500 µL diluted HEK293T cell lysate. IP was performed according to the GFP-Trap manual and bound EGFP was eluted with 2x SDS-sample buffer. Input (I), flow through (FT), and bound (B) fractions were analyzed by SDS-PAGE and Western blot (WB). SDS-PAGE was stained with InstantBlue™. WB was stained with GFP antibody rabbit polyclonal [PABG1] (ChromoTek) and anti-rabbit secondary antibody HRP. Note that a shorter exposure time was used for the WB analysis of 5 µg and 0.63 µg EGFP.
Increase the concentration of the POI
If it is not possible to increase the abundancy or expression level of the POI, then its concentration in the assay may be increased using the tips and tricks below:
- Use more cells or combine several cell pellets for the preparation of the cell lysate.
- Do not add dilution buffer to the cell lysate or add only a small volume of buffer.
Note: It is still recommended to use 500 µL cell lysate and 25 µL of GFP-Trap slurry.
Note: More frequent and/or harsher washing may be needed.
-
Elute with 20-50 µL 2x SDS-sample buffer and try to load as much of the elution fraction on the SDS-PAGE/WB as possible.
Amplify the signal of the POI in WB
If the amount of POI in the elution fraction is very low, it may be insufficient for WB detection, even if the IP was successful. In this case, it is recommended to amplify the WB signal of the POI. The highest signal amplification is obtained when a polyclonal primary antibody in combination with a polyclonal secondary antibody is used for WB detection.
