MODified™ Histone Peptide Array
MODified-Histone-Peptide-Array-AM668
Synonyms
| Name | Format | Cat No. | Price |
|---|
Overview
The MODified Histone Peptide Array* is a valuable research tool that can be used to screen antibodies, enzymes and proteins for cross-reactivity or binding interactions with histones and their post-translational modifications (PTM). The MODified Histone Peptide Arrays screen 59 acetylation, methylation, phosphorylation, and citrullination modifications on the N-terminal tails of histones H2A, H2B, H3 and H4. Each peptide array contains 384 unique histone modification combinations in duplicate, including up to four separate modifications on the same 19mer peptide, offering the most extensive coverage available for commercial arrays of similar format. This extensive coverage of histone modifications enables the study of not only individual sites, but also the effects of neighboring modifications on recognition and binding. The unique synthesis and conjugation methods ensure greater than 95% purity of bound peptides and high peptide density at each spot, which is advantageous for analysis of interaction sites with low binding constants. The simple array protocol works like a Western blot; no special equipment needed.
Active Motif offers everything needed to study histone PTM interactions.
*CelluSpots™ arrays are manufactured under license by INTAVIS Bioanalytical Instruments AG and sold through Active Motif as MODified™ Histone Peptide Arrays.
Documents (9)
Data
MODified™ Histone Peptide Array
The MODified Histone Peptide Array is designed to study the binding specificity of antibodies, enzymes and proteins. The arrays are glass slides that contain up to four histone post-translational modifications per peptide to allow for analysis of individual histone modifications as well as the effects of neighboring modifications. The MODified Array Labeling Kit and the MODified Protein Domain Binding Kit are complementary products containing the buffers and reagents required to process the peptide array for chemiluminescent detection. Please see the table below for the combination of reagents recommended for each application.
| Application | Array | Labeling Kit |
|---|---|---|
| Antibody Specificity | MODified™ Histone Peptide Array | Array Labeling Kit |
| Enzymatic Effects | MODified™ Histone Peptide Array | Array Labeling Kit |
| Protein Domain Specificity | MODified™ Histone Peptide Array | Protein Domain Binding Kit |
Evaluation of Antibody Binding Specificity
Active Motif uses the Histone Peptide Array to help validate the binding specificity of our histone modification antibodies. Select antibody images and graphs are available below. To see a complete list of all the Active Motif antibodies that have been tested using the MODified™ Histone Peptide Array, please follow the link to array tested.

Figure 1: Image of ECL detection of Histone H3 trimethyl Lys9 (H3K9me3) pAb.
Active Motif's Histone H3 trimethyl Lys9 (H3K9me3) pAb (Catalog No. 39161) was used at 1:2,000 dilution on the MODified Histone Peptide Array. Anti-rabbit HRP secondary antibody was used at 1:2500 dilution, followed by ECL detection and image capture with a CCD camera.

Figure 2: Graphical analysis of Histone H3 trimethyl Lys9 (H3K9me3) pAb.
Spot densitometry was used to analyze spot intensity from the ECL camera image. Results were compared to the provided reference grid to determine the associated peptide content. The results were graphed as a specificity factor, which is the ratio of the average intensity of all spots containing the mark divided by the ratio of the average intensity of all spots not containing the mark. The results show Active Motif's Histone H3 trimethyl Lys9 pAb (Catalog No. 39161) has very little cross-reactivity with other histone modifications.

Figure 3: Image of ECL detection of Histone H3 dimethyl Lys4 (H3K4me2) pAb.
Active Motif's Histone H3 dimethyl Lys4 (H3K4me2) pAb (Catalog No. 39141) was used at 1:10,000 dilution on the MODified Histone Peptide Array. Anti-rabbit HRP secondary antibody was used at 1:2500 dilution, followed by ECL detection and image capture with a CCD camera.

Figure 4: Graphical analysis of Histone H3 dimethyl Lys4 (H3K4me2) pAb.
Spot densitometry was used to analyze spot intensity from the ECL camera image. Results were compared to the provided reference grid to determine the associated peptide content. The results were graphed as a specificity factor, which is the ratio of the average intensity of all spots containing the mark divided by the ratio of the average intensity of all spots not containing the mark. The results show Active Motif's Histone H3 dimethyl Lys4 pAb (Catalog No. 39141) has very little cross-reactivity with other histone modifications.
Evaluation of Protein Domain Binding
The MODified Histone Peptide Array was used in combination with the MODified Protein Domain Binding Kit to evaluate the binding specificity of the Tudor domain protein, Jumonji-domain containing protein 2A (JMJD2A). Tudor domain-containing proteins are known to bind to methylated lysine or arginine residues on Histones H3 & H4.

Figure 5: Graphical analysis of the JMJD2A protein domain binding specificity.
The His-tagged Tudor domain protein, JMJD2A, was used at a 50 nM concentration with the MODified Protein Domain Binding Kit and the MODified Histone Peptide Array. An image of the array was captured using a CCD camera. Graphical analysis of selected data from the Array Analyze softtware program which was used to calculate the spot intensity of the binding events for the JMJD2A protein domain. The data shows the specificity of the JMJD2A protein domain for Histone H4K20me3 and H3K27me3 (green arrow). The presence of additional modifications with the same peptide had varying influences on spot intensity. There was relatively little change when the H3K27me3 modification was present together with modifications at R26 (copper arrows). But, when the S28ph modification was adjacent to K27me3, binding of the JMJD2A protein domain was inhibited greatly (red arrows).
Application Data using the LI-COR® Odyssey® Infrared Imaging System
The MODified Histone Peptide Array was analyzed using the LI-COR Odyssey imaging system in a dual detection assay. The LI-COR images were kindly provided courtesy of Dr. Anil Panigrahi at Baylor College of Medicine, Houston, TX.

Figure 6: Analysis of the MODified Histone Peptide Array using the LI-COR Odyssey Infrared Imaging System.
The MODified Histone Peptide Array was incubated with anti-H3K9me3 pAb (Active Motif Cat. No. 39765) for 6 hours at a 1:2000 dilution using LI-COR Odyssey Blocking Buffer. The array was washed with TBST and then incubated with anti-H3K27me2me3 mAb (Active Motif Cat. No. 39538) overnight at a 1:2000 dilution in LI-COR Odyssey Blocking Buffer. Following washes in TBST, the array was incubated with a 1:1000 dilution of goat anti-rabbit IRDye800 and a 1:5000 dilution of goat anti-mouse IRDye680 for 45 minutes. The array was washed. One ml TBS was dropped onto the glass surface of the LI-COR Odyssey Imaging System and the array was gently laid over the TBS to prevent trapping air bubbles. The array was scanned and the image was captured with medium intensity (a setting of 4.5 for both 700 and 800 channels) and medium resolution (169 µm). The top image shows the binding pattern of the H3K9me3 antibody alone, the middle image shows the binding pattern of the H3K27me2me3 antibody in combination with the c-Myc control antibody from the Array Labeling Kit (Active Motif Cat. No. 13006) and the bottom image is the composite from the Odyssey system. The results show that the LI-COR Odyssey Infrared Imaging system can be used for detection of the MODified Histone Peptide Arrays and that dual detection is possible.
MODified Method
MODified™ Histone Peptide Array Advantages
- Extensive coverage - 384 unique modification combinations are represented in duplicate, including as many as four modifications on each peptide
- High quality peptides - Synthesis method ensures >95% purity
- Easy to read - Array contains control peptides and reference marks
- Detects like a Western blot - works with either ECL-based or colorimetric detection systems
- Versatility - use the arrays to study antibody, protein or enzyme interactions
How do the MODified™ Histone Peptide Arrays Work?
To generate MODified Arrays, Active Motif synthesizes a series of 19mer histone H2A, H2B, H3 and H4 peptides, each of which may contain as many as four modifications per peptide, on a modified cellulose support which can be dissolved after synthesis. The solutions of peptides covalently linked to macromolecular cellulose are then spotted in duplicate onto a glass slide as a three-dimensional layer. This high peptide density in the spots is advantageous for identifying protein-interaction sites with low binding constants. The peptide coated slides contain 768 spots (384 modification combinations in duplicate) and are offered as single arrays (Catalog No. 13001) or as a pack of five arrays (Catalog No. 13005).
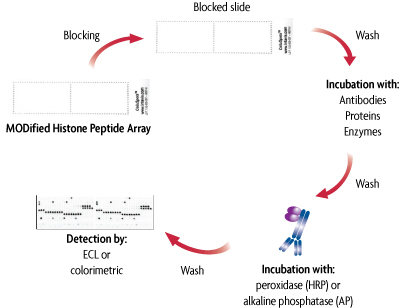
The MODified Histone Peptide Arrays can be used to screen antibodies, enzymes and proteins for cross reactivity or binding interactions with histones and their post-translational modifications. Following a blocking step, the array is incubated with either antibodies, proteins or enzymes for one hour, washed and then incubated for one hour with either a horseradish peroxidase (HRP) or alkaline phosphatase (AP) conjugated secondary antibody. Developing solution is then added and the image is captured using film or a CCD camera; no special equipment is needed. The MODified Histone Peptide Arrays can also be analyzed using the LI-COR® Odyssey® infrared imaging system and infrared detection reagents (see Figure 6 under the Data tab).
Figure 1: Flow chart of the MODified Histone Peptide Array.
This flow chart illustrates the use of the MODified Histone Peptide Arrays (Click image to enlarge.)
MODified Histone Peptide Array Analysis
Active Motif's FREE Array Analyze Software can be used to analyze the spot intensities on the array and generate a graphical analysis of the histone peptide modification interactions. The Excel file containing the list of histone modifications and their associated locations has been incorporated into the software to help automate the identification process for the arrays. With the Array Analyze Software, information about spot intensity, averages and errors can be saved in Excel-compatible files to allow for individual analysis. Additionally, up to three individual histone modifications can be displayed in superposition to the experimental data, enabling better visualization of the effects of neighboring modifications. Click here to download the Array Analyze Software Manual and the FREE Array Analyze Software.
MODified Histone Peptide Array Specifications:
- Standard microscope slides (26x76 mm, white coating)
- 768 spots per slide (384 peptide-conjugate spots printed in duplicate)
- Spot-to-spot distance 1.2 mm
- Peptides are covalently bound to cellulose via C-terminus
- Arrays contain control peptides and location marks
To download a list of histone modifications offered on the MODified Histone Peptide array and the corresponding peptide location on the array, CLICK HERE.
Contents
Contents & Storage
MODified Histone Peptide Array
The MODified Histone Peptide Arrays can be stored at 4°C for up to three months. For long-term storage of up to six months, we recommend keeping the arrays at -20°C. The arrays are available individually or in packs of 5 arrays.
MODified Array Labeling Kit
The MODified Array Labeling Kits are shipped on dry ice and contain reagents with multiple storage temperatures inside. Please store each component at the temperature indicated below. This kit includes the following components:
- Blocking Buffer AM2; Store at -20°C
- c-Myc mAb; Store at -20°C
- 10X Wash Buffer AM4; Store at 4°C
- Anti-mouse HRP-conjugated secondary antibody; Store at 4°C
- Anti-rabbit HRP-conjugated secondary antibody; Store at 4°C
- ECL Reagent A; Store at 4°C
- ECL Reagent B; Store at 4°C
MODified Protein Domain Binding Kit
The MODified Protein Domain Binding Kits are shipped on dry ice and contain reagents with multiple storage temperatures inside. Please store each component at the temperature indicated below. This kit includes the following components:
- Recombinant His-G9a protein; Store at -80°C
- Blocking Buffer AM2; Store at -20°C
- Interaction Buffer AM1; Store at -20°C
- 1 M DTT; Store at -20°C
- Anti-His6-tag antibody; Store at -20°C
- 10X Wash Buffer AM4; Store at 4°C
- Anti-mouse HRP-conjugated secondary antibody; Store at 4°C
- ECL Reagent A; Store at 4°C
- ECL Reagent B; Store at 4°C