Gelshift™ Chemiluminescent EMSA
Gelshift-Chemiluminescent-EMSA-AM648
Synonyms
| Name | Format | Cat No. | Price |
|---|
Overview
The Gelshift Chemiluminescent EMSA Assay Kit provides a simple, non-radioactive assay to identify protein-DNA binding with proven reagents. In this electrophoretic mobility shift assay (EMSA), cell extracts or purified factors are incubated with biotin end-labeled probe containing the consensus binding site of interest. Samples in which the protein of interest bound the target DNA will migrate slower than DNA alone resulting in a "shift" of the labeled DNA band. For complete details, click the EMSA Method tab below.
The Gelshift Chemiluminescent EMSA Assay Kit includes all the reagents needed to study protein-DNA binding for your sample system. The kit also includes biotin-labeled and unlabeled control DNA and control nuclear extract. Click the Gel Shift tab below for data and more information; kit manuals can be downloaded under the Documents tab.
For those that wish to make their own Gel shift / Supershift Assay, we offer specialized binding buffers with differing properties to best fit the transcription factor you are interested in. These buffers are not used in the Gelshift Chemiluminescent EMSA Assay Kit.
EMSA Method
Gelshift Chemiluminescent EMSA Method
The Gelshift Chemiluminescent EMSA Kit provides a non-radioactive method to detect DNA-protein interactions. In this method, cell extracts or purified factor are incubated with a biotin 3' or 5' end-labeled DNA probe containing the consensus binding site of interest. Samples are then resolved by electrophoresis on a native polyacrylamide gel and transferred to a nylon membrane. The biotin end-labeled DNA probe is detected using streptavidin conjugated to horseradish peroxidase (HRP) and a chemiluminescent substrate. Samples in which the protein of interest bound the target DNA will migrate slower than DNA alone resulting in a "shift" of the labeled DNA band.
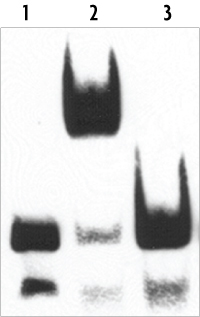
Example EMSA Binding Reactions
- Biotin-labeled DNA = no shift in the absence of cell extract or purified factor
- Biotin-labeled DNA + Extract = DNA shift due to the binding of protein to the labled DNA probe
- Biotin-labeled DNA + Extract + Excess Unlabeled DNA = no shift as the excess of unlabeled DNA competes for binding of the target protein in the extract; this reaction verifies the specificity of the protein-DNA interaction
Gelshift Chemiluminescent EMSA Advantages
- Non-radioactive assay
- Better sensitivity than radioactive or digoxigenin methods
- Fast procedure can be completed in 5 hours
- Additional reagents supplied to help optimize conditions for your sample system
- Includes control DNA and extract to help new users understand the methods as they optimize conditions for their sample systems
Gel Shift
Gelshift or Electrophoretic Mobility Shift (EMSA) Info
Electrophoretic mobility shift assays (EMSA), also known as gel shifts, gel retardation assays or mobility assays can be used to study DNA-protein interactions1-3. The principle behind EMSA relies on the fact that DNA-protein complexes migrate slower than DNA alone in a native polyacrylamide or agarose gel. This difference in electrophoretic separation of DNA-protein complexes can be visualized as a "shift" in migration of the labeled DNA band. This enables researches to screen different nuclear extracts or gene promoters for specific transcription factor DNA binding activity.
Better sensitivity
The non-radioactive format of the Gelshift Chemiluminescent EMSA Assay Kit does not sacrifice sensitivity when compared to 32P or digoxigenin methods.
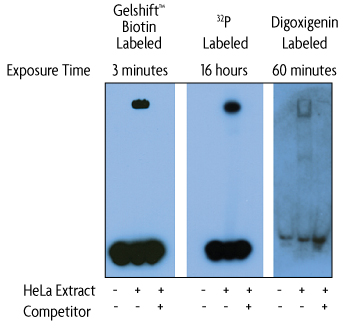
Figure 1: Gelshift Chemiluminescent EMSA Kit provides better sensitivity.
HeLa nuclear extract (6.8 µg) was incubated with 20 fmol Oct-1 duplex DNA labeled for use in either the Gelshift Chemiluminescent EMSA kit, a digoxigenin-based EMSA or with 32P using T4 polynucleotide kinase (40,000 cpm/reaction) for use in a radioactive EMSA. The kits were used according to the manufacturer's instructions. The radioactive EMSA was exposed directly to X-ray film using screens.

Figure 2: Obtain sensitive results for the DNA-protein complex of interest.
The Gelshift Chemiluminescent EMSA Kit was used to obtain sensitive gel shift results for four different DNA-protein complexes. Biotin-labeled target duplexes were incubated with HeLa nuclear extract (Oct-1, AP1 and NFκB) or the provided Control Nuclear Extract from the kit. Unlabeled competitor sequences were used at 200-fold molar excess over labeled DNA. The Control reactions from the Epstein-Barr Nuclear Antigen (EBNA) system were supplemented with 2.5% glycerol and 0.05% NP-40, while the AP1 reactions were supplemented with 10% glycerol. X-ray film exposure time varied from 2 minutes for the EBNA Control, Oct-1 and AP1 to 5 minutes for NFκB.
References
1. Fried, M. and Crothers, D.M. (1981) Nucleic Acids Res. 9: 6505-6525.
2. Revzin, A. (1989) BioTechniques 7: 346-354.
3. Hendrickson, W. (1985) BioTechniques 3: 198-207.
Binding Buffers
Active Motif's Supershift and Gel shift Buffers have been shown to significantly reduce non-specific background. These buffers are available for purchase to save you the time and inconvenience of optimizing buffers for your own experiments.
Binding Buffers B-1 through B-4 are for incubation of the extract:antibody mixture, while Binding Buffers C-1 through C-3 facilitate binding of the labeled probe.
These buffers are not used with the Gelshift Chemiluminescent EMSA.
| Buffer | Description |
|---|---|
| Buffer B-1 | Higher pH, poly D(I/C) |
| Buffer B-2 | Neutral pH, poly D(I/C) |
| Buffer B-3 | Neutral pH, poly D(I/C) + detergent |
| Buffer B-4 | Higher pH, Herring Sperm DNA |
| Buffer C-1 | Higher pH, no blocking DNA |
| Buffer C-2 | Neutral pH, no blocking DNA |
| Buffer C-3 | Neutral pH, no blocking DNA, detergent |
Contents
Contents & Storage
The Gelshift Chemiluminescent EMSA Kits contain 10X Binding Buffer, Biotin Control DNA, Unlabeled Control DNA, Control Nuclear Extract, Poly d(I-C), 50% Glycerol, 1% NP-40, 1 M KCl, 100 mM MgCl2, 200 mM EDTA pH 8.0 and 5X Loading Buffer, which are all stored at -20°C. Additionally, the kit includes Streptavidin HRP-conjugate, Chemiluminescent Reagent, Reaction Buffer, Blocking Buffer, 4X Wash Buffer and Substrate Equilibration Buffer which should be stored at 4°C.
